Market Definition
The market refers to the industry focused on the research, development, manufacturing, and commercialization of peptide-based drugs for various medical applications. Peptides are short chains of amino acids that play crucial roles in biological functions and are used in treating diseases such as cancer, metabolic disorders, infectious diseases, and neurological conditions.
The report examines critical market drivers, industry trends, and regional analysis, along with the regulatory frameworks that will impact the pace of market growth over the forecast period.
Peptide Therapeutics Market Overview
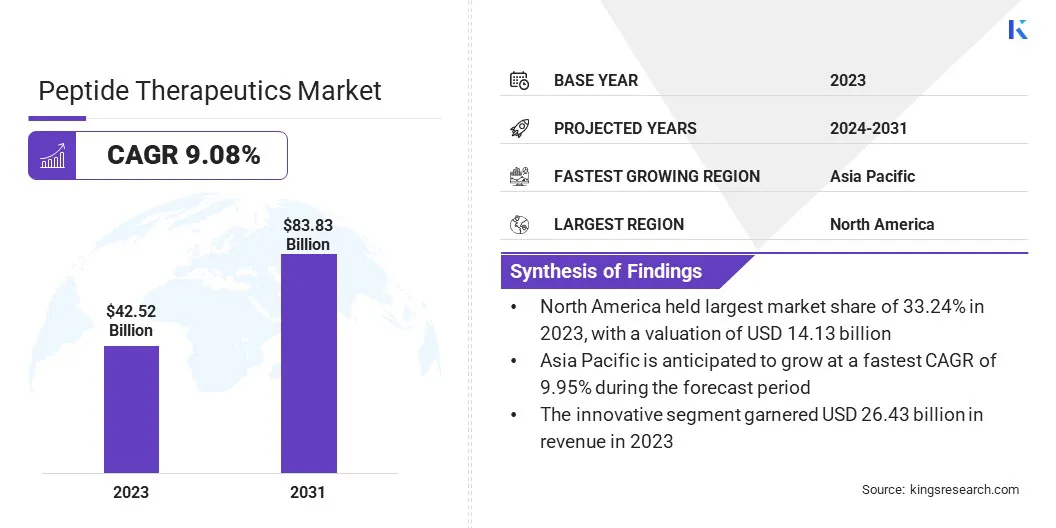
The global peptide therapeutics market size was valued at USD 42.52 billion in 2023 and is projected to grow from USD 45.61 billion in 2024 to USD 83.83 billion by 2031, with a CAGR of 9.08% during the forecast period.
The market is driven by advancements in drug development and increasing demand for targeted therapies. Peptides, known for their high specificity and efficacy, are widely used in treating conditions such as cancer, metabolic disorders, cardiovascular diseases, and infectious diseases. The rising prevalence of chronic illnesses, along with ongoing innovations in peptide synthesis and formulation technologies, is fueling market expansion.
Major companies operating in the peptide therapeutics industry are AbbVie Inc., Novartis AG, Pfizer Inc., Eli Lilly and Company, F. Hoffmann-La Roche Ltd, Novo Nordisk A/S, PeptiDream Inc., Takeda Pharmaceutical Company Limited, Bachem AG, Assia Chemical Industries Ltd, AstraZeneca, CordenPharma, Debiopharm, GlaxoSmithKline plc., and Hanmi Pharm.Co., Ltd.
Additionally, the growing adoption of biologics and personalized medicine is enhancing the role of peptide-based drugs in modern therapeutics, in turn, further driving the growth of the market. The expanding application of peptides in drug delivery systems and the integration of AI-driven drug discovery processes are also contributing to the sector's rapid evolution.
Furthermore, increased investment in biotechnology and pharmaceutical R&D, coupled with regulatory support for peptide-based drug approvals, is accelerating market growth.
- In May 2023, Fujitsu launched the "Biodrug Design Accelerator" platform to enhance peptide drug discovery research by streamlining the design, synthesis, testing, and analysis (DMTA) process. The platform, developed in collaboration with PeptiDream Inc., aims to accelerate peptide drug development through process visualization and centralized data management.
Key Highlights
- The peptide therapeutics industry size was valued at USD 42.52 billion in 2023.
- The market is projected to grow at a CAGR of 9.08% from 2024 to 2031.
- North America held a market share of 33.24% in 2023, with a valuation of USD 14.13 billion.
- The innovative segment garnered USD 26.43 billion in revenue in 2023.
- The parenteral segment is expected to reach USD 34.66 billion by 2031.
- The gastrointestinal disorders segment is expected to reach USD 23.97 billion by 2031.
- The liquid-phase peptide synthesis (LPPS) segment is expected to reach USD 22.47 billion by 2031.
- The market in Asia Pacific is anticipated to grow at a CAGR of 9.95% during the forecast period.
Market Driver
Expansion of Peptide Manufacturing and Advancements in Drug Delivery
The market is experiencing significant growth, driven by the expansion of peptide manufacturing capabilities and advancements in peptide drug delivery technologies. The increasing demand for inhalable biologics has led to substantial investments in large-scale peptide production, enabling the efficient synthesis of complex peptides with enhanced purity, stability, and scalability.
This expansion is crucial for meeting the growing needs of pharmaceutical companies developing inhalable biologic therapies for respiratory and systemic diseases. Additionally, advancements in peptide drug delivery technologies such as lipid-based nanoparticles, microsphere formulations, and advanced dry powder inhalers (DPIs) are improving peptide stability and efficiency in pulmonary absorption.
Moreover, they increase bioavailability and enable more precise dosing, reduce side effects, and improve patient adherence. These factors are accelerating the adoption of inhalable biologics, expanding their therapeutic applications beyond respiratory conditions to include metabolic and autoimmune disorders.
- In March 2024, Cytovance Biologics and PolyPeptide announced their collaboration to enhance the development and manufacturing of microbial and mammalian-expressed peptide drugs. The partnership combines Cytovance’s expertise in biologic process development and cGMP manufacturing with PolyPeptide’s extensive capabilities in peptide synthesis and scale-up, providing an integrated solution for drug developers.
Market Challenge
High Production Costs and Scalability Challenges
A major challenge in the peptide therapeutics market is the high production costs and scalability limitations associated with manufacturing peptide-based drugs. Peptides are complex molecules that require precise synthesis methods, such as solid-phase peptide synthesis (SPPS) and recombinant DNA technology.
These processes demand specialized reagents, purification techniques, and high-end equipment, leading to significant operational costs. Additionally, several therapeutic peptides are highly sensitive and prone to degradation, aggregation, or poor stability, making large-scale production a challenging factor for market entrants.
To address these challenges, the industry is leveraging advanced manufacturing techniques like hybrid synthesis methods (which combine SPPS with liquid-phase peptide synthesis for efficiency), continuous manufacturing, and automated purification processes. These approaches help improve production, reduce waste, and optimize resource utilization, in turn, lowering production costs.
Market Trend
Personalization and AI-Driven Drug Discovery
The market is undergoing rapid advancements, primarily driven by the growing focus on personalized peptide therapies stems from the increasing need for precision medicine, particularly in treating complex diseases like cancer, metabolic disorders, and autoimmune conditions.
Traditional therapies often follow a one-size-fits-all approach, which may not be effective for all patients. In contrast, personalized peptide therapeutics are designed to target specific molecular pathways based on a patient’s genetic profile, disease subtype, and biomarker data.
This enhances treatment efficacy, reduces side effects, and improves overall patient outcomes. With advancements in biomarker identification and sequencing technologies, peptide-based therapies can be tailored for individualized treatment strategies, further increasing their adoption in clinical settings.
Additionally, the adoption of AI and computational tools in peptide drug discovery is transforming the efficiency and success rate of drug development. AI-driven platforms utilize machine learning algorithms, molecular modeling, and predictive analytics to rapidly screen and optimize peptide candidates.
These tools help analyze vast biological datasets, predict peptide-receptor interactions, and refine molecular structures to enhance stability and bioavailability. AI also enables virtual simulations of peptide behavior, reducing reliance on labor and time-intensive laboratory experiments.
As a result, drug discovery timelines are significantly shortened, and the success rates of peptide therapeutics in clinical development are improved. This technological advancement is driving innovation in peptide therapeutics, making drug development more cost-effective and efficient.
- In January 2025, Pepticom secured USD 6.6 million in Series A1 funding to advance its AI-driven peptide drug discovery platform. The company is developing oral IL-17 inhibitors targeting autoimmune diseases like psoriasis and psoriatic arthritis. Pepticom’s AI platform accelerates peptide design, enhancing stability and therapeutic potential while reducing drug discovery timelines.
Peptide Therapeutics Market Report Snapshot
|
Segmentation
|
Details
|
|
By Type
|
Generic, Innovative
|
|
By Route of Administration
|
Parenteral, Oral, Others
|
|
By Application
|
Gastrointestinal Disorders, Neurological Disorders, Metabolic Disorders, Cancer, Others
|
|
By Technology
|
Solid Phase Peptide Synthesis (SPPS), Recombinant DNA, Hybrid, Liquid-Phase Peptide Synthesis (LPPS), Others
|
|
By Region
|
North America: U.S., Canada, Mexico
|
|
Europe: France, UK, Spain, Germany, Italy, Russia, Rest of Europe
|
|
Asia-Pacific: China, Japan, India, Australia, ASEAN, South Korea, Rest of Asia-Pacific
|
|
Middle East & Africa: Turkey, UAE, Saudi Arabia, South Africa, Rest of Middle East & Africa
|
|
South America: Brazil, Argentina, Rest of South America
|
Market Segmentation
- By Type (Generic, Innovative): The generic segment earned USD 16.09 billion in 2023 due to increasing demand for cost-effective peptide drugs and patent expirations of branded therapeutics.
- By Route of Administration (Parenteral, Oral, Others): The parenteral held 41.79% of the market in 2023, due to the high bioavailability and rapid absorption of injectable peptide drugs.
- By Application (Gastrointestinal Disorders, Neurological Disorders, Metabolic Disorders, Cancer, Others): The gastrointestinal disorders segment is projected to reach USD 23.97 billion by 2031, owing to the rising prevalence of digestive diseases and the growing adoption of peptide-based treatments.
- By Technology (Solid Phase Peptide Synthesis (SPPS), Recombinant DNA, Hybrid, Liquid-Phase Peptide Synthesis (LPPS), Others): The liquid-phase peptide synthesis (LPPS) segment is projected to reach USD 22.47 billion by 2031, owing to its efficiency in large-scale peptide production and cost-effectiveness.
Peptide Therapeutics Market Regional Analysis
Based on region, the market has been classified into North America, Europe, Asia Pacific, Middle East & Africa, and Latin America.
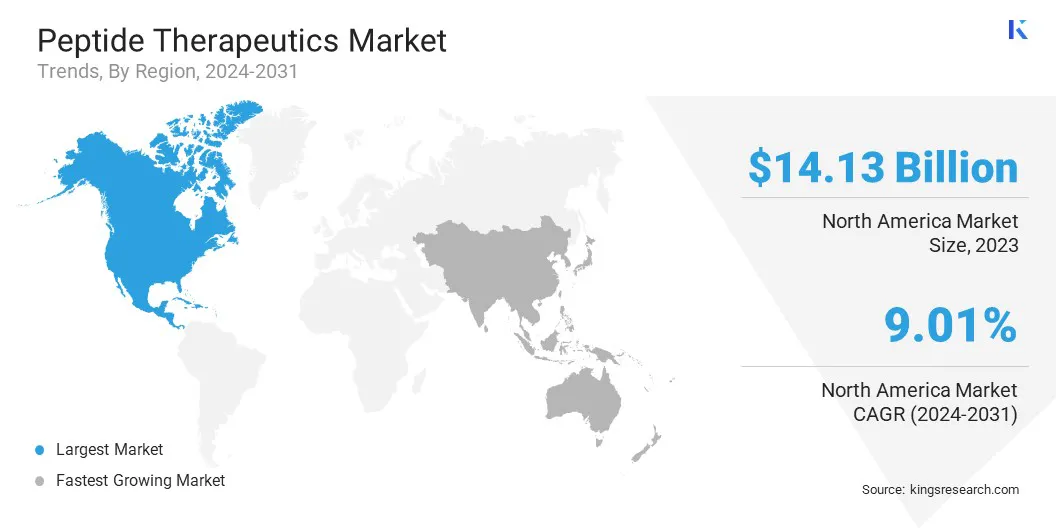
North America accounted for 33.24% share of the peptide therapeutics market in 2023, with a valuation of USD 14.13 billion. The region’s dominance is largely attributed to its robust biopharmaceutical sector, extensive R&D activities, and the presence of major market players.
The United States, in particular, is a key contributor, supported by high healthcare expenditure and advanced drug discovery technologies. Moreover, the increasing adoption of peptide-based drugs for the treatment of oncology, metabolic, and neurological disorders is further driving the growth of the market.
Additionally, increasing FDA approvals for novel peptide therapeutics along with the rising demand for biologics have fueled market growth. Collaborations between academic institutions and pharmaceutical companies further strengthen North America’s position in the market.
- In July 2024, CordenPharma invested USD 973 million to expand its peptide technology across the U.S. and Europe. The expansion includes the construction of a large-scale peptide manufacturing facility in Colorado, USA, and a greenfield site in Europe to support peptide drug development from early clinical to commercial stages.
The peptide therapeutics industry in Asia Pacific is expected to register the fastest growth in the market, with a projected CAGR of 9.95% over the forecast period. This growth is driven by increasing healthcare investments, government support for biopharmaceutical innovation, and rising demand for targeted therapies.
Countries such as China, India, Japan, and South Korea are leading contributors, benefiting from low-cost manufacturing, advancements in peptide synthesis technologies, and a growing pharmaceutical industry. The rising prevalence of chronic diseases, including diabetes, cardiovascular disorders, and cancer is boosting the adoption of peptide-based drugs in this region.
Additionally, improving healthcare infrastructure and increasing clinical trial activities are further accelerating market expansion. Many multinational pharmaceutical companies are also outsourcing peptide drug manufacturing to Asia Pacific due to cost advantages, further driving the region’s growth in the global market.
Regulatory Frameworks
- In the US, the regulatory body for peptide therapeutics is the Food and Drug Administration (FDA), specifically its Center for Drug Evaluation and Research (CDER), which ensures the safety, efficacy, and quality of these drugs.
- In Europe, the European Medicines Agency (EMA) is the primary regulatory body for peptide therapeutics, ensuring the safety, efficacy, and quality of these medicines through a coordinated network with national authorities.
- In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA), under the Ministry of Health, Labour, and Welfare (MHLW), is the regulatory authority responsible for reviewing and approving pharmaceuticals, including peptide therapeutics, ensuring their safety, efficacy, and quality.
- In India, the Central Drugs Standard Control Organisation (CDSCO) regulates peptide therapeutics, and they require applicants to submit applications as a subsequent new drug for approval of synthetically manufactured peptides, considering safety and efficacy.
Competitive Landscape
The peptide therapeutics market is characterized by continuous innovation, and strategic collaborations among key players. Companies are focusing on advanced peptide synthesis technologies, including solid-phase peptide synthesis (SPPS), liquid-phase peptide synthesis (LPPS), and hybrid approaches, to enhance production efficiency and scalability.
Leading firms are investing heavily in research and development (R&D) to introduce next-generation peptide drugs with improved efficacy and bioavailability. Strategic partnerships with biotechnology firms, research institutions, and contract manufacturing organizations (CMOs) are becoming increasingly common to accelerate drug development and commercialization.
Companies are also focusing on expanding their intellectual property portfolios through patents on novel peptide formulations and drug delivery technologies. Additionally, key players are adopting mergers and acquisitions to strengthen their market position and expand product pipelines.
- In June 2023, IRBM extended its collaboration with Merck & Co. Inc. to advance research in peptide therapeutics, focusing on the development of orally available peptide drug candidates. The partnership leverages IRBM’s expertise in peptide design, synthesis, and drug discovery technologies, including phage display peptide libraries and advanced synthetic strategies, alongside Merck’s drug development capabilities.
List of Key Companies in Peptide Therapeutics Market:
- AbbVie Inc.
- Novartis AG
- Pfizer Inc.
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd
- Novo Nordisk A/S
- PeptiDream Inc.
- Takeda Pharmaceutical Company Limited
- Bachem AG
- Assia Chemical Industries Ltd
- AstraZeneca
- CordenPharma
- Debiopharm
- GlaxoSmithKline plc.
- Hanmi Pharm.Co., Ltd.
Recent Developments (Acquisition/ Agreements/Collaboration)
- In March 2025, AbbVie and Gubra went into a license agreement for the development of GUB014295, a long-acting amylin analog peptide for obesity treatment. AbbVie will lead the global development and commercialization of the therapy, leveraging Gubra's expertise in peptide-based drug discovery.
- In October 2024, Amneal Pharmaceuticals, Inc. and Metsera, Inc. collaborated to develop and supply next-generation medicines for obesity and metabolic diseases. The partnership focuses on the large-scale manufacturing of GLP-1 and amylin receptor agonists, including ultra-long-acting injectable and oral peptide-based therapies. As part of the agreement, Amneal will construct new peptide synthesis and sterile fill-finish manufacturing facilities in India, while also supporting Metsera in product development and commercialization in emerging markets.
- In July 2024, AstraZeneca completed the acquisition of Amolyt Pharma, a biotechnology company specializing in rare endocrine diseases. The acquisition strengthens AstraZeneca’s rare disease portfolio with eneboparatide (AZP-3601), a Phase III investigational therapeutic peptide for hypoparathyroidism, designed to regulate calcium levels and improve kidney function.
- In April 2024, PeptiDream expanded its collaboration with Novartis to advance peptide drug discovery, focusing on radionuclide-peptide conjugates (RI-PDCs) for targeted radioligand therapy. The partnership leverages PeptiDream’s Peptide Discovery Platform System platform technology to develop novel cyclic peptides that selectively deliver radioactive materials to cancer cells, minimizing damage to healthy tissue.
, efficacy,


