Market Definition
The market encompasses the diagnosis, treatment, and management of pain resulting from nerve damage or dysfunction caused by conditions such as diabetes, shingles, multiple sclerosis, spinal cord injuries, and chemotherapy-induced neuropathy.
This market includes pharmaceutical interventions such as anticonvulsants, antidepressants, opioids, and topical treatments, as well as non-pharmacological approaches like neuromodulation, physical therapy, and regenerative medicine.
Neuropathic Pain Market Overview
The global neuropathic pain market size was valued at USD 7.81 billion in 2023 and is projected to grow from USD 8.33 billion in 2024 to USD 14.10 billion by 2031, exhibiting a CAGR of 7.81% during the forecast period.
The market is registering significant growth, due to the rising prevalence of chronic pain conditions associated with diabetes, cancer, multiple sclerosis, and spinal cord injuries. The increasing geriatric population, which is more susceptible to nerve-related pain disorders, further drives the market.
Advancements in drug development, including novel anticonvulsants, antidepressants, and biologics, are enhancing treatment efficacy, while growing investments in regenerative medicine and gene therapy offer promising long-term solutions.
Major companies operating in the neuropathic pain industry are Sun Pharmaceutical Industries Ltd., Novartis AG, Abbott, Pfizer Inc, Almatica Pharma LLC., Grünenthal, Azurity Pharmaceuticals, Inc., Accord Healthcare, Eli Lilly and Company, GLENMARK PHARMACEUTICALS LTD., Vertex Pharmaceuticals Incorporated, Assertio Holdings, Inc., Catalent, Inc, Neuropathix, Inc., and Collegium Pharmaceutical.
Additionally, the increasing adoption of personalized medicine and digital health solutions, such as AI-powered pain management platforms and remote monitoring, is improving patient outcomes and optimizing treatment strategies. The growing awareness of neuropathic pain and improved access to healthcare services, especially in emerging economies, are further fueling the market.
- In January 2023, Abbott announced FDA approval for its Proclaim XR spinal cord stimulation (SCS) system to treat painful diabetic peripheral neuropathy (DPN). This non-medication, non-opioid treatment provides chronic pain relief and integrates with NeuroSphere Virtual Clinic, allowing remote therapy adjustments.

Key Highlights:
- The neuropathic pain industry size was valued at USD 7.81 billion in 2023.
- The market is projected to grow at a CAGR of 7.81% from 2024 to 2031.
- North America held a market share of 49.94% in 2023, with a valuation of USD 3.90 billion.
- The anticonvulsant segment garnered USD 2.77 billion in revenue in 2023.
- The diabetic neuropathy segment is expected to reach USD 7.12 billion by 2031.
- The retail pharmacies segment is expected to reach USD 7.19 billion by 2031.
- The market in Asia Pacific is anticipated to grow at a CAGR of 8.95% during the forecast period.
Market Driver
Rising Incidence of Chronic Conditions
The neuropathic pain market is registering significant growth, driven by the rising incidence of diabetes and other chronic conditions that contribute to nerve-related pain. Pharmaceutical companies are investing in innovative treatment solutions to enhance patient outcomes.
The demand for combination therapies is gaining traction, due to their ability to provide improved efficacy by targeting multiple pain pathways simultaneously. Regulatory approvals for such novel formulations are further accelerating their adoption, creating opportunities for market expansion.
Additionally, advancements in drug formulations and increased awareness among healthcare professionals and patients are supporting the uptake of these treatments.
- In February 2023, Anglo-French Drugs & Industries Ltd. launched AFD-NP, a Nortriptyline and Pregabalin combination for moderate to severe neuropathic pain. Approved by the Drug Control Department, the product aims to address the growing burden of neuropathic pain, particularly due to diabetes.
Market Challenge
Limited Efficacy and Side Effects
Key challenges in the neuropathic pain market are the limited efficacy and side effects of existing drug therapies, leading to suboptimal patient adherence and treatment outcomes. Many commonly prescribed medications, such as antidepressants and anticonvulsants, often provide partial pain relief while causing adverse effects like dizziness, drowsiness, and gastrointestinal issues.
Additionally, the long-term use of opioids raises concerns over dependency and regulatory restrictions, further complicating treatment options. A potential solution lies in the advancement of non-pharmacological and targeted treatment approaches, such as neuromodulation therapies, precision medicine, and novel drug formulations with improved safety profiles.
Increased investment in biologics, gene therapy, and non-invasive nerve stimulation technologies can offer more effective and personalized treatment options.
Market Trend
Non-invasive and Non-pharmacological Treatment Approaches
The market is registering a shift toward non-invasive and non-pharmacological treatment approaches, driven by advancements in medical technology and the increasing demand for alternatives to traditional drug therapies.
Amid rising concerns over the long-term use of pharmacological treatments, particularly for chronic conditions like Painful Diabetic Neuropathy (PDN), interest in innovative solutions such as peripheral nerve stimulation and neuromodulation techniques is gaining traction.
These advanced therapies offer effective pain relief while minimizing side effects, improving patient compliance, and enhancing long-term treatment outcomes.
Regulatory approvals and strong clinical validation are further accelerating their adoption, fostering confidence among healthcare providers and expanding their integration into pain management protocols. Market players are increasingly investing in R&D to refine these technologies as the preference for non-invasive treatments grows.
- In January 2024, Neuralace Medical, Inc. announced FDA clearance for Axon Therapy (mPNS), the first non-invasive magnetic peripheral nerve stimulation treatment for Painful Diabetic Neuropathy (PDN). A randomized controlled trial demonstrated significant pain reduction and improved patient outcomes, marking a breakthrough in non-pharmacological pain management.
Neuropathic Pain Market Report Snapshot
|
Segmentation
|
Details
|
|
By Type
|
Antidepressants (Tricyclic Antidepressants, Serotonin–Noradrenaline Reuptake Inhibitors (SNRI)), Anticonvulsant, Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), Opioids, Others
|
|
By Indication
|
Diabetic Neuropathy, Postherpetic Neuralgia, Trigeminal Neuralgia, Chemotherapy-Induced Peripheral Neuropathy (CIPN), Others
|
|
By Distribution Channel
|
Hospital Pharmacies, Retail Pharmacies, Online Pharmacies
|
|
By Region
|
North America: U.S., Canada, Mexico
|
|
Europe: France, UK, Spain, Germany, Italy, Russia, Rest of Europe
|
|
Asia-Pacific: China, Japan, India, Australia, ASEAN, South Korea, Rest of Asia-Pacific
|
|
Middle East & Africa: Turkey, UAE, Saudi Arabia, South Africa, Rest of Middle East & Africa
|
|
South America: Brazil, Argentina, Rest of South America
|
Market Segmentation:
- By Type (Antidepressants, Anticonvulsant, Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), Opioids, and Others): The anticonvulsant segment earned USD 2.77 billion in 2023, due to its widespread use as a first-line treatment for neuropathic pain, particularly in conditions like diabetic neuropathy and postherpetic neuralgia.
- By Indication (Diabetic Neuropathy, Postherpetic Neuralgia, Trigeminal Neuralgia, and Chemotherapy-Induced Peripheral Neuropathy (CIPN)): The diabetic neuropathy segment held 49.39% share of the market in 2023, due to the rising global prevalence of diabetes, increasing cases of nerve damage associated with prolonged hyperglycemia, and growing awareness of early diagnosis and management of diabetic neuropathic pain.
- By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies): The retail pharmacies segment is projected to reach USD 7.19 billion by 2031, owing to the increasing accessibility of over-the-counter and prescription neuropathic pain medications, expanding pharmacy networks in emerging economies, and the growing consumer preference for retail pharmacies on account of their convenience & personalized consultation services.
Neuropathic Pain Market Regional Analysis
Based on region, the global market has been classified into North America, Europe, Asia Pacific, Middle East & Africa, and Latin America.
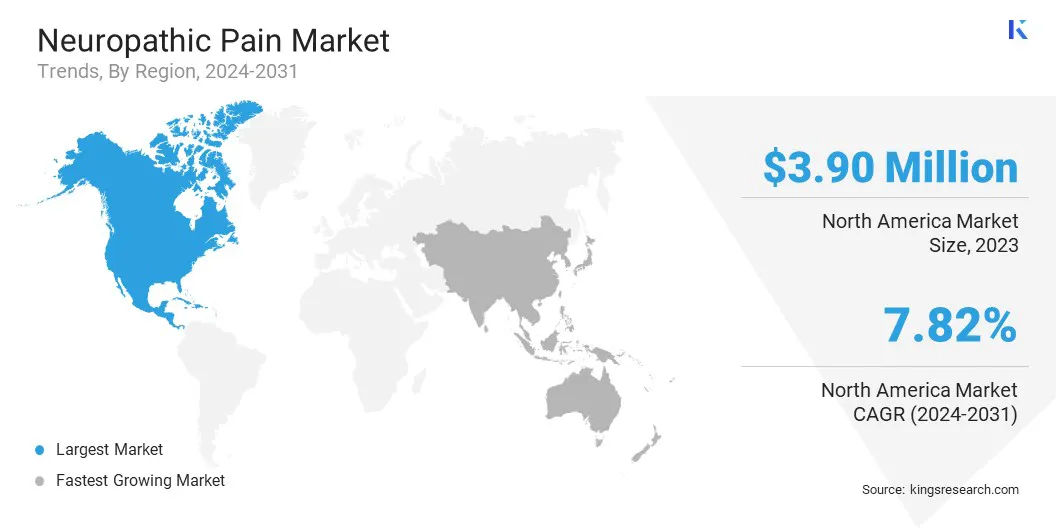
North America accounted for 49.94% share of the neuropathic pain market in 2023, with a valuation of USD 3.90 billion. The market in the region is driven by the high prevalence of chronic diseases such as diabetes and cancer, which are major contributors to neuropathic pain.
Additionally, strong healthcare infrastructure, advanced R&D capabilities, and rapid adoption of novel pain management therapies further support market expansion. The increasing availability of FDA-approved drugs and non-invasive treatment options, along with rising patient awareness, is accelerating product demand.
North America remains a key region for innovation and investment in neuropathic pain treatment, due to continuous advancements in neuromodulation technologies and non-opioid therapeutics in the region. The presence of leading pharmaceutical companies and research institutions in the region further strengthens the market’s competitive landscape.
- In February 2025, Vistagen announced that the U.S. Patent and Trademark Office (USPTO) granted a patent for its oral non-opioid product candidate, AV-101, for the treatment of neuropathic pain. The patent, which will remain valid until at least 2034, is part of Vistagen’s global patent portfolio covering manufacturing methods and therapeutic uses of AV-101 for disorders involving the NMDA receptor. The company aims to explore strategic collaborations to advance the clinical development and commercialization of AV-101 as a potential non-opioid alternative for pain and dyskinesia.
The market in Asia Pacific is poised to grow at a significant CAGR of 8.95% over the forecast period, driven by the rising prevalence of diabetes, cancer, and other chronic conditions leading to neuropathic pain. Increasing healthcare investments, expanding pharmaceutical manufacturing, and improving access to advanced pain management therapies are fueling the market.
Additionally, the growing geriatric population, which is more susceptible to neuropathic pain, is driving the demand for effective treatment solutions. Governments in countries like China, India, and Japan are actively promoting the development of healthcare infrastructure and encouraging the adoption of cost-effective generic drugs and innovative therapies.
Furthermore, rising awareness, increasing clinical research activities, and the entry of global pharmaceutical players into the region are accelerating market expansion.
Regulatory Frameworks
- In the U.S., the Food and Drug Administration (FDA) regulates the approval, safety, efficacy, manufacturing, labeling, and marketing of drugs and medical devices used for neuropathic pain treatment. The FDA ensures that prescription medications, including antidepressants, anticonvulsants, NSAIDs, and opioids, meet stringent clinical and quality standards before reaching the market.
- In Europe, the market is regulated at multiple levels. The European Medicines Agency (EMA) oversees the approval, safety, and monitoring of pharmaceuticals, ensuring that drugs used for neuropathic pain treatment meet stringent efficacy and safety standards.
Competitive Landscape
The neuropathic pain industry is characterized by the presence of well-established pharmaceutical companies, medical device manufacturers, and emerging biotechnology firms focused on developing innovative pain management solutions. Leading players dominate the market with a strong portfolio of neuropathic pain medications.
The competition is driven by continuous R&D efforts aimed at improving drug efficacy, reducing side effects, and exploring novel therapeutic targets such as sodium channel blockers and monoclonal antibodies. In addition to pharmaceutical advancements, neuromodulation is gaining traction, with investments in next-generation spinal cord stimulators and peripheral nerve stimulation devices.
Strategic partnerships, mergers, and acquisitions are common in this competitive landscape, as companies seek to expand their product pipelines and global reach. Collaborations between pharmaceutical firms and digital health startups are driving the integration of AI-powered pain management platforms, remote patient monitoring, and wearable pain relief technologies.
Moreover, regulatory approvals for new drug formulations, combination therapies, and non-opioid alternatives are shaping market dynamics, with an increasing emphasis on opioid-free pain management solutions due to the global opioid crisis.
- In November 2024, Grünenthal and Averitas Pharma completed Phase III trial recruitment for QUTENZA in post-surgical neuropathic pain (PSNP). Topline results are expected in Q4 2025, with a sNDA submission planned for 2026, aiming to expand QUTENZA’s non-opioid pain treatment indications in the U.S.
List of Key Companies in Neuropathic Pain Market:
- Sun Pharmaceutical Industries Ltd.
- Novartis AG
- Abbott
- Pfizer Inc
- Almatica Pharma LLC.
- Grünenthal
- Azurity Pharmaceuticals, Inc.
- Accord Healthcare
- Eli Lilly and Company
- GLENMARK PHARMACEUTICALS LTD.
- Vertex Pharmaceuticals Incorporated
- Assertio Holdings, Inc.
- Catalent, Inc
- Neuropathix, Inc.
- Collegium Pharmaceutical
Recent Developments (Approvals)
- In January 2025, Vertex Pharmaceuticals Incorporated announced that the U.S. Food and Drug Administration (FDA) approved JOURNAVX, a first-in-class, non-opioid NaV1.8 pain signal inhibitor for the treatment of adults with moderate-to-severe acute pain. JOURNAVX is the first new class of pain medicine approved in over 20 years and is indicated for use across all types of acute pain without evidence of addictive potential. The approval marks a historic milestone in acute pain management, offering an alternative to opioids.
- In April 2024, Vertex Pharmaceuticals advanced its suzetrigine (VX-548) pain program, with the FDA granting a rolling NDA submission for acute pain and Breakthrough Therapy designation for DPN. A Phase 3 program for DPN will begin in 2H 2024, and Phase 2 enrollment for LSR is set to complete by year-end.


