Market Definition
Topical drug delivery refers to administering drugs directly onto the skin or mucous membranes to achieve targeted therapeutic effects. It uses creams, gels, ointments, sprays, and patches to treat localized conditions or deliver systemic therapy efficiently. The market encompasses development, manufacturing, and commercialization of formulations for dermatology, pain management, and other therapeutic areas.
Topical Drug Delivery Market Overview
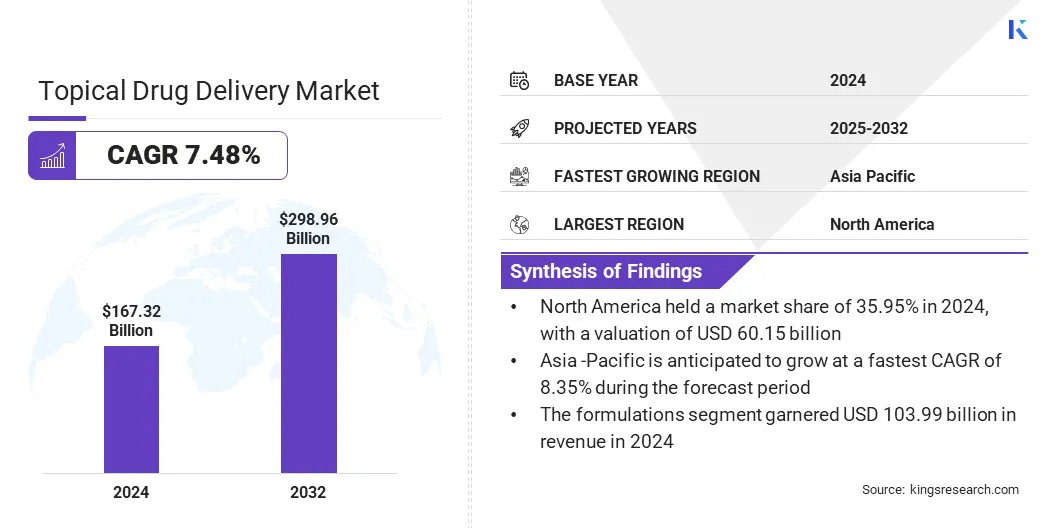
The global topical drug delivery market size was valued at USD 167.32 billion in 2024 and is projected to grow from USD 179.50 billion in 2025 to USD 298.96 billion by 2032, exhibiting a CAGR of 7.48% over the forecast period.
Market growth is driven by the rising prevalence of skin disorders and chronic conditions, creating strong demand for localized therapies such as creams, ointments, gels, and sprays. The increasing adoption of transdermal patches for controlled and systemic drug delivery is further supporting their use across dermatology, pain management, and chronic disease treatment.
Key Highlights:
- The topical drug delivery industry size was recorded at USD 167.32 billion in 2024.
- The market is projected to grow at a CAGR of 7.48% from 2025 to 2032.
- North America held a share of 35.95% in 2024, valued at USD 60.15 billion.
- The formulations segment garnered USD 103.99 billion in revenue in 2024.
- The dermal segment is expected to reach USD 78.51 billion by 2032.
- The hospitals segment is anticipated to witness the fastest CAGR of 33.24% over the forecast period.
- Asia Pacific is anticipated to grow at a CAGR of 8.35% over the forecast period.
Major companies operating in the topical drug delivery market are Bayer AG, Galderma SA, Novartis AG, Glenmark Pharmaceuticals Ltd, Johnson & Johnson, Almirall S.A., AbbVie Inc., GlaxoSmithKline plc, Bausch Health Companies Inc., Hisamitsu Pharmaceutical Co., Inc., Cipla Limited, Viatris Inc., Solventum, Organon & Co., and Teva Pharmaceuticals USA, Inc.
Growing concerns over UV exposure and environmental factors are boosting demand for topical treatments such as sunscreens and targeted therapies. Additionally, the rising prevalence of skin cancer is creating increased demand for advanced topical chemotherapeutics and targeted treatment options.
- The American Cancer Society (ACS) projects that an estimated 104,960 new cases of melanoma will be diagnosed in the U.S. in 2025.
Market Driver
Increasing Prevalence of Dermatological Conditions
A key factor propelling the growth of the topical drug delivery market is the increasing prevalence of dermatological conditions such as acne, eczema, psoriasis, and rosacea. Pharmaceutical companies are focusing on the development of targeted and effective topical therapies to manage these skin disorders.
Dermatologists and healthcare providers are recommending non-invasive topical treatments to improve patient outcomes and minimize systemic side effects. This rising focus on skin health is accelerating demand for advanced topical formulations and driving market growth.
- In February 2025, the American Academy of Dermatology Association reported that acne is the most common skin condition in the U.S., affecting up to 50 million people annually.
Market Challenge
High Development and Manufacturing Costs
A key challenge limiting the growth of the topical drug delivery market is the high development and manufacturing costs of advanced formulations. Developing transdermal patches, nanocarriers, and controlled-release systems requires specialized equipment and extensive preclinical and clinical testing. Moreover, strict quality control and regulatory compliance further increase production expenses, thereby limiting market growth.
To address this challenge, market players are investing in advanced manufacturing technologies and automation to improve efficiency and reduce production expenses.
They are adopting modular and scalable platforms for transdermal patches, nanocarriers, and controlled-release systems to accelerate development timelines. They are forming strategic collaborations with contract research and manufacturing organizations (CROs and CMOs) to accelerate development timelines and improve production efficiency.
Market Trend
Development of Novel Topical Formulations
A key trend influencing the topical drug delivery market is the development of novel topical formulations. Manufacturers are creating advanced gels, creams, and transdermal systems that are improving drug stability, enhancing skin penetration, and limit the amount of drug entering the bloodstream thereby reducing systemic side effects.
These innovations are targeting chronic and complex conditions such as neuropathic pain, inflammatory skin disorders, and viral infections. These formulations are enabling localized and precise therapy while enhancing patient adherence and optimizing therapeutic outcomes.
- In April 2025, Lyka Labs secured an Indian patent for Pregabalin Gel 8%. The gel is a novel topical formulation designed to manage diabetic neuropathic pain.
Topical Drug Delivery Market Report Snapshot
|
Segmentation
|
Details
|
|
By Product
|
Formulations (Solid, Semi-Solid, Liquid), Devices (Transdermal Patches, Inhalers & Nebulizers, Metered-Dose Sprayers, Others)
|
|
By Route of Administration
|
Dermal, Ophthalmic, Nasal, Rectal, Others
|
|
By End User
|
Hospitals, Specialty Clinics & Dermatology Centers, Ambulatory Surgical Centers, Home-Care Settings
|
|
By Region
|
North America: U.S., Canada, Mexico
|
|
Europe: France, UK, Spain, Germany, Italy, Russia, Rest of Europe
|
|
Asia-Pacific: China, Japan, India, Australia, ASEAN, South Korea, Rest of Asia-Pacific
|
|
Middle East & Africa: Turkey, U.A.E., Saudi Arabia, South Africa, Rest of Middle East & Africa
|
|
South America: Brazil, Argentina, Rest of South America
|
Market Segmentation:
- By Product (Formulations, and Devices): The formulations segment earned USD 103.99 billion in 2024, driven by rising demand for creams, gels, ointments, and transdermal patches for localized and systemic therapies.
- By Route of Administration (Dermal, Ophthalmic, Nasal, Rectal, and Others): The dermal segment held 26.23% of the market in 2024, supported by high prevalence of skin disorders and growing adoption of topical treatments.
- By End User (Hospitals, Specialty Clinics & Dermatology Centers, Ambulatory Surgical Centers, and Home-Care Settings): The hospitals segment is projected to reach USD 98.44 billion by 2032, owing to increased hospital-based treatments and widespread availability of advanced topical drug delivery systems.
Topical Drug Delivery Market Regional Analysis
Based on region, the market has been classified into North America, Europe, Asia Pacific, Middle East & Africa, and South America.
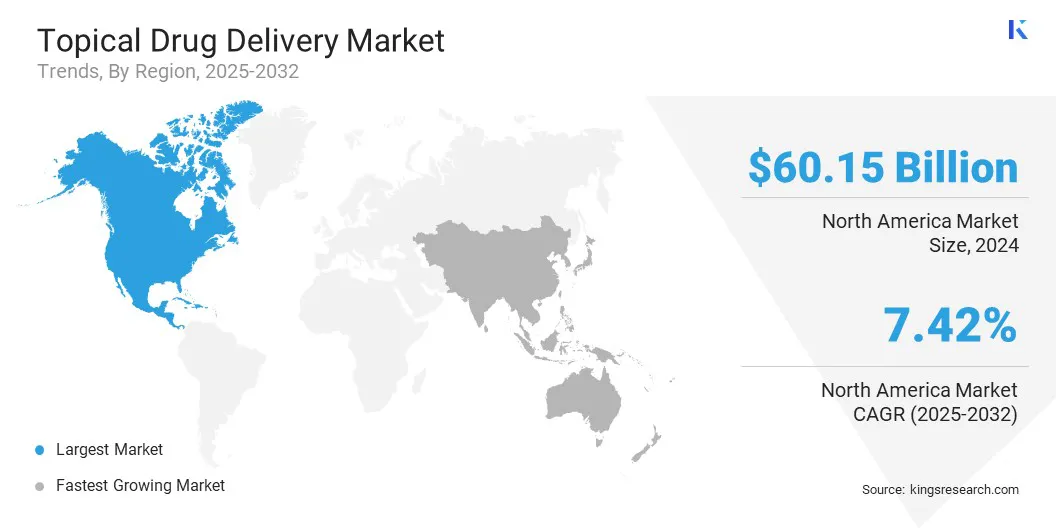
North America topical drug delivery market share stood at 35.95% in 2024, valued at USD 60.15 billion. This dominance is driven by the high prevalence of dermatological conditions such as acne, eczema, and psoriasis in the U.S., coupled with strong healthcare access in the region. Increasing cases of skin cancer are fueling demand for advanced topical chemotherapeutics to provide targeted treatment and improve patient outcomes.
The surge in chronic diseases such as diabetes is further boosting the need for specialized topical therapies to manage complications and enhance patient care. Additionally, the presence of well-established contract development and manufacturing organizations (CDMOs) in the region is driving faster development of innovative topical and transdermal products.
- In July 2024, MedPharm, Ltd. merged with Tergus Pharma to form a leading topical and transdermal CDMO with end-to-end development, clinical trial manufacturing, and commercial production capabilities. The merger expands the company’s global presence in the UK and the U.S. and strengthens its capabilities in hormone-based and highly potent drug manufacturing.
Asia Pacific is set to grow at a CAGR of 8.35% over the forecast period. This growth is attributed to the increasing prevalence of skin disorders and rapid population growth across the region. Rising consumer awareness about sun protection and skincare is encouraging the adoption of sunscreens and dermal treatments.
Growing awareness of skin cancer prevention is creating demand for advanced topical chemotherapeutics. Additionally, regional players are expanding pharmaceutical R&D infrastructure, enabling the development of innovative topical formulations, thereby boosting market growth.
- In January 2025, Emcure Pharmaceuticals inaugurated a state-of-the-art Formulation R&D Centre near Tapovan Circle, Ahmedabad. The centre is designed to advance complex drug delivery systems, including advanced dermal therapies.
Regulatory Frameworks
- In the U.S., the Food and Drug Administration (FDA) regulates the market by overseeing product approvals, clinical trials, labeling, and post-market surveillance. It ensures the safety and quality of creams, gels, ointments, and transdermal patches while enforcing GMP compliance and reviewing applications through New Drug Application (NDA) pathways.
- In the UK, the Medicines and Healthcare Products Regulatory Agency (MHRA) regulates the market by evaluating clinical trials, granting market authorizations, and monitoring safety. It ensures the quality, safety, and therapeutic performance of topical formulations. The MHRA also enforces GMP compliance and post-market surveillance under U.K.-specific regulations.
- In China, the National Medical Products Administration (NMPA) regulates the market by supervising drug registration, clinical evaluations, and manufacturing standards. It ensures safety, efficacy, and quality of topical and transdermal formulations. The NMPA also enforces GMP compliance and oversees imported drugs under China’s Drug Administration Law framework.
- In India, the Central Drugs Standard Control Organization (CDSCO) regulates the market by approving new drugs, monitoring clinical trials, and licensing manufacturers. It ensures the quality, safety, and efficacy of formulations under the Drugs and Cosmetics Act. CDSCO also enforces GMP compliance and coordinates with state-level regulatory authorities.
Competitive Landscape
Companies operating in the topical drug delivery industry are actively developing innovative formulations such as sustained-release gels, liposomal creams, and medicated transdermal patches to improve efficacy and patient compliance.
They are expanding research and development capabilities to create advanced therapies such as hormone-based gels and localized analgesic formulations for dermatological conditions. Additionally, players are focusing on strategic collaborations to accelerate the development of specialized topical treatments for rare and complex skin disorders.
- In May 2025, Dermaliq and DEBRA Research entered into a strategic collaboration to advance cutaneous drug delivery for Epidermolysis Bullosa (EB). The partnership will focus on developing topical treatments for wound healing, itch relief, and prophylactic therapies.
Top Key Companies in Topical Drug Delivery Market:
- Bayer AG
- Galderma SA
- Novartis AG
- GLENMARK PHARMACEUTICALS LTD
- Johnson & Johnson
- Almirall, S.A
- AbbVie Inc
- GlaxoSmithKline plc
- Bausch Health Companies Inc.
- Hisamitsu Pharmaceutical Co., Inc.
- Cipla Limited
- Viatris Inc
- Solventum
- Organon & Co
- Teva Pharmaceuticals USA, Inc.
Recent Developments
- In July 2025, Pelthos Therapeutics launched ZELSUVMI (berdazimer) Topical Gel 10.3%, the first FDA-approved at-home treatment for molluscum contagiosum. The gel allows patients to apply treatment outside clinical care.
- In October 2024, Organon acquired Dermavant, including VTAMA (tapinarof) Cream 1%, a non-steroidal topical therapy for plaque psoriasis. This acquisition expands Organon’s dermatology portfolio.


