Market Definition
The market encompasses a range of pharmaceutical interventions designed to treat sepsis, a life-threatening condition caused by the body's extreme response to infection.
This market includes various classes of antibiotics and therapies that target the underlying bacterial infections and manage sepsis-related complications. The report outlines major factors driving the market, along with regional analysis and regulatory frameworks that are set to influence its growth trajectory over the forecast period.
Sepsis Therapeutics Market Overview
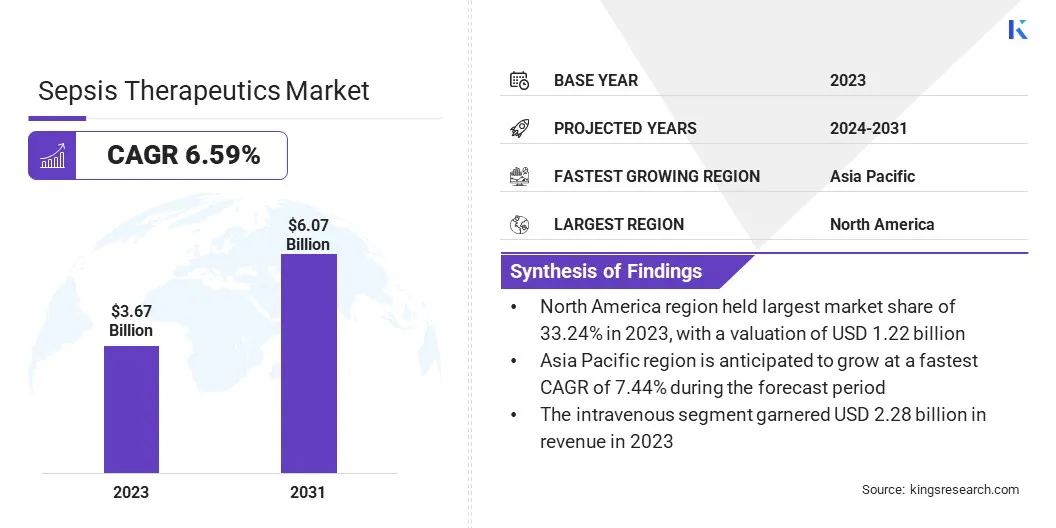
The global sepsis therapeutics market size was valued at USD 3.67 billion in 2023 and is projected to grow from USD 3.88 billion in 2024 to USD 6.07 billion by 2031, exhibiting a CAGR of 6.59% during the forecast period.
The market is driven by the increasing global incidence of sepsis, rising hospitalization rates, and advancements in antimicrobial therapies. The growing elderly population, which is more susceptible to infections, along with the rising prevalence of chronic diseases such as diabetes and cancer, has heightened the demand for effective sepsis treatment options.
Major companies operating in the sepsis therapeutics industry are Fresenius Kabi USA, GlaxoSmithKline plc., Cytovale, Inc., Eli Lilly and Company, Lupin, F. Hoffmann-La Roche Ltd, Pfizer Inc., Cadila Pharmaceuticals, Innoviva Specialty Therapeutics, and Teva Pharmaceutical Industries Ltd.
The increasing adoption of precision medicine and biomarker-based diagnostics is enabling faster and more targeted treatment approaches. Strategic collaborations between pharmaceutical companies and research institutions are further accelerating drug development, fostering innovation, and expanding the availability of advanced sepsis therapeutics globally.
- In May 2024, Sanofi, Formation Bio, and OpenAI are partnering to develop AI-driven software, streamlining drug development and accelerating patient access to new medicines. This first-of-its-kind collaboration in pharma and life sciences integrates data, software, and tailored AI models. Sanofi will leverage proprietary data to enhance AI capabilities, advancing its vision of becoming the first AI-powered biopharma at scale.
Key Highlights:
- The sepsis therapeutics industry size was valued at USD 3.67 billion in 2023.
- The market is projected to grow at a CAGR of 6.59% from 2024 to 2031.
- North America held a market share of 33.24% in 2023, with a valuation of USD 1.22 billion.
- The cephalosporin segment garnered USD 1.31 billion in revenue in 2023.
- The intravenous segment is expected to reach USD 3.74 billion by 2031.
- The hospital pharmacies segment is expected to reach USD 2.34 billion by 2031.
- The market in Asia Pacific is anticipated to grow at a CAGR of 7.44% during the forecast period.
Market Driver
Rising Incidence of Sepsis and Hospital-acquired Infections
The market is registering significant growth, driven by the rising incidence of sepsis and hospital-acquired infections (HAIs), along with advancements in antibiotic development and precision medicine.
The increasing prevalence of HAIs, coupled with the growing burden of antimicrobial resistance, has led to a surge in sepsis cases globally, necessitating effective therapeutic interventions. Additionally, an aging population and a rising number of immunocompromised patients further contribute to the increasing demand for advanced sepsis treatments.
- In June 2024, the World Health Organization (WHO) published its latest report on antibacterial agents, including antibiotics, in global clinical and preclinical development. The clinical pipeline expanded from 80 agents in 2021 to 97 in 2023, boosting the need for innovative solutions to combat serious infections and replace treatments losing efficacy due to widespread usage.
Market Challenge
Antimicrobial Resistance
Rising antimicrobial resistance (AMR) poses a major challenge to the sepsis therapeutics market, which significantly limits the effectiveness of existing antibiotics used for sepsis treatment. The overuse and misuse of antibiotics have led to the emergence of multidrug-resistant (MDR) bacterial strains, making it increasingly difficult to treat sepsis effectively and increasing mortality rates.
A potential solution to this issue is the promotion of antibiotic stewardship programs to ensure the responsible use of antibiotics, coupled with increased government and private sector investments in next-generation antimicrobial agents.
Additionally, the integration of AI-driven drug discovery can accelerate the identification and development of effective antibiotics, addressing the urgent need for new sepsis therapeutics.
Market Trend
integration of Artificial Intelligence (AI)
The market is registering significant advancements, driven by the increasing adoption of rapid diagnostic tools and the integration of AI in sepsis management. The development of advanced molecular diagnostics and biomarker-based detection technologies is enhancing early diagnosis, enabling faster and more targeted antibiotic administration, which is crucial for improving survival rates.
Additionally, AI-powered solutions are transforming the way sepsis is detected and treated by analyzing electronic health records (EHRs), vital signs, and laboratory data to predict sepsis risk in real time.
AI-driven drug discovery is also accelerating the development of novel antimicrobial agents and optimizing treatment regimens, leading to more personalized and effective therapeutic strategies. Such innovations in healthcare systems position the market for significant growth, with improved patient outcomes and enhanced treatment efficiency.
- In April 2024, Prenosis, Inc. announced that the U.S. Food and Drug Administration (FDA) had granted marketing authorization for its Sepsis ImmunoScore through the De Novo pathway. This AI-powered software as a medical device (SaMD) is designed to enhance the early detection and prediction of sepsis, making it the first FDA-approved AI diagnostic tool for this complex condition.
Sepsis Therapeutics Market Report Snapshot
|
Segmentation
|
Details
|
|
By Drug Class
|
Cephalosporin, Aminoglycosides, Glycopeptide, Others
|
|
By Route of Administration
|
Intravenous, Oral
|
|
By Distribution Channel
|
Hospital Pharmacies, Retail Pharmacies, Online
|
|
By Region
|
North America: U.S., Canada, Mexico
|
|
Europe: France, UK, Spain, Germany, Italy, Russia, Rest of Europe
|
|
Asia-Pacific: China, Japan, India, Australia, ASEAN, South Korea, Rest of Asia-Pacific
|
|
Middle East & Africa: Turkey, UAE, Saudi Arabia, South Africa, Rest of Middle East & Africa
|
|
South America: Brazil, Argentina, Rest of South America
|
Market Segmentation:
- By Drug Class (Cephalosporin, Aminoglycosides, Glycopeptide, Others): The cephalosporin segment earned USD 1.31 billion in 2023, due to its broad-spectrum efficacy, widespread use in empirical sepsis treatment, and lower resistance rates compared to other antibiotic classes.
- By Route of Administration (Intravenous, Oral): The intravenous segment held 62.15% share of the market in 2023, due to its rapid drug delivery, higher bioavailability, and preference for treating severe sepsis cases in hospital settings.
- By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online): The hospital pharmacies segment is projected to reach USD 2.34 billion by 2031, owing to the high hospitalization rate of sepsis patients, the critical need for immediate antibiotic administration, and the growing presence of well-equipped healthcare facilities.
Sepsis Therapeutics Market Regional Analysis
Based on region, the global market has been classified into North America, Europe, Asia Pacific, Middle East & Africa, and Latin America.
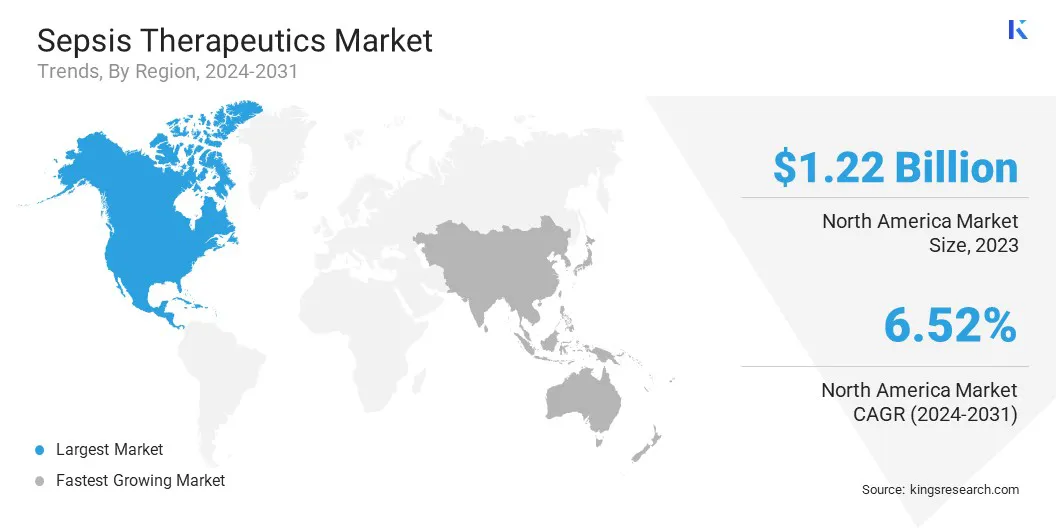
North America accounted for 33.24% share of the sepsis therapeutics market in 2023, with a valuation of USD 1.22 billion. This dominance is attributed to the region's well-established healthcare infrastructure, high healthcare expenditure, and advanced diagnostic capabilities that facilitate early sepsis detection and treatment.
The widespread availability of advanced antibiotics, strong presence of leading pharmaceutical companies, and significant investment in Research and Development (R&D) have further contributed to market growth. Additionally, the rising prevalence of HAIs, increasing incidence of chronic diseases, and a growing elderly population have driven the demand for effective sepsis therapeutics in the region.
The market in Asia Pacific is poised to grow at a significant CAGR of 7.44% over the forecast period, driven by expanding healthcare infrastructure, increasing awareness of sepsis management, and a rising patient pool.
The region's growing population, particularly in China, India, and Japan, has led to a higher incidence of infectious diseases, fueling the demand for antibiotics such as cephalosporins, aminoglycosides, and glycopeptides. Additionally, government initiatives to improve critical care services, increasing healthcare investments, and the rising number of sepsis-related hospitalizations are propelling the market.
The rapid adoption of advanced medical technologies and the presence of emerging pharmaceutical manufacturers are also contributing to the market’s growth in Asia Pacific.
Regulatory Frameworks
- In the U.S., the Food and Drug Administration (FDA) regulates sepsis therapeutics under the New Drug Application (NDA) and Biologics License Application (BLA) pathways, ensuring that all antimicrobial agents used for sepsis treatment meet stringent safety, efficacy, and quality standards.
- In Europe, the European Medicines Agency (EMA) regulates sepsis therapeutics through the Committee for Medicinal Products for Human Use (CHMP), ensuring that all antimicrobial agents meet strict safety, efficacy, and quality requirements before market approval.
Competitive Landscape
The sepsis therapeutics industry is characterized by intense competition, with key players focusing on strategic collaborations, drug development, and market expansion to strengthen their positions. Companies are heavily investing in R&D to introduce next-generation antibiotics and novel therapies that address antimicrobial resistance.
Many players are leveraging strategic partnerships with research institutions and biotech firms to accelerate drug discovery and clinical trials. Major pharmaceutical firms are actively engaging in mergers and acquisitions to enhance market reach, allowing them to expand their product portfolios and gain access to advanced technologies.
Additionally, regulatory approvals and fast-track designations are being pursued to bring innovative sepsis therapeutics to the market more efficiently. Companies are also increasing their presence in emerging markets through strategic alliances and distribution agreements to tap into the growing demand for sepsis treatment.
- In April 2024, AdrenoMed AG secured Fast Track designation from the U.S. FDA for enibarcimab, its non-neutralizing monoclonal antibody designed for septic shock treatment. The company is advancing toward a confirmatory Phase IIb/III clinical trial to assess enibarcimab’s efficacy in reducing septic shock mortality through a precision medicine approach.
List of Key Companies in Sepsis Therapeutics Market:
Recent Developments (Product Launch)
- In March 2025, CLEW announced the launch of its Sepsis Virtual Unit (SVU), an AI-driven clinical surveillance solution aimed at enhancing sepsis detection, improving SEP-1 compliance, and optimizing patient outcomes. The SVU provides real-time monitoring and surveillance, allowing clinicians to identify sepsis earlier, facilitate timely interventions, and streamline adherence to evolving regulatory requirements.
- In August 2023, Cytovale commercially launched the IntelliSep sepsis test in the U.S. following FDA 510(k) clearance in December 2022. The Lake Regional Medical Center in Baton Rouge, Louisiana, became the first medical center to adopt IntelliSep for emergency department use, enabling rapid sepsis diagnosis with results in under 10 minutes.


