Market Definition
The peptide cancer vaccine market involves the development and application of peptide-based immunotherapies designed to stimulate the body’s immune system to target and destroy cancer cells. These vaccines utilize specific tumor-associated peptides to induce a precise cytotoxic T-cell response for improved cancer control and treatment outcomes.
The report includes segmentation based on type, application, and region. Key treatment areas such as breast, lung, prostate, and melanoma cancers rely on peptide cancer vaccines to enable targeted therapy, reduce tumor recurrence, and enhance patient survival rates.
Peptide Cancer Vaccine Market Overview
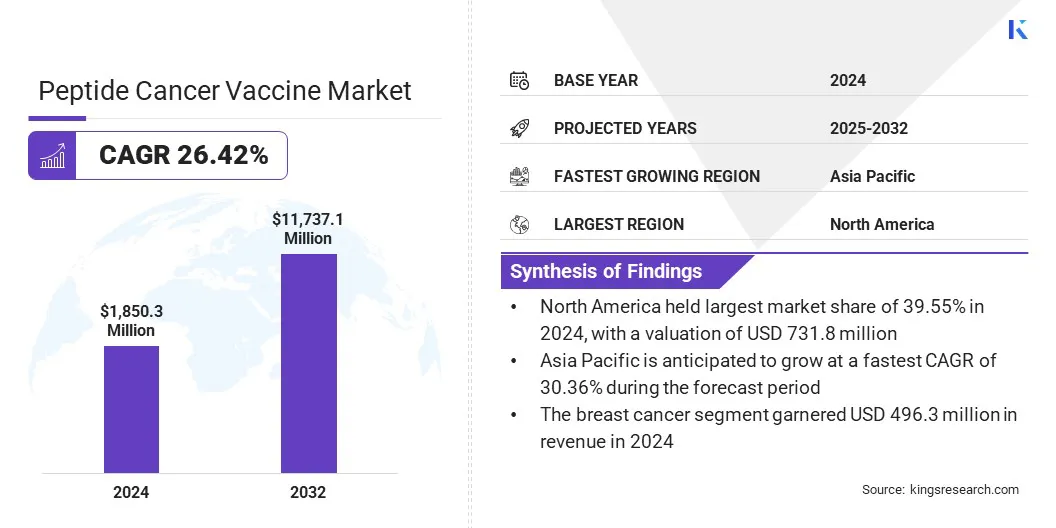
The global peptide cancer vaccine market size was valued at USD 1,850.30 million in 2024 and is projected to grow from USD 2,274.13 million in 2025 to USD 11,737.11 million by 2032, exhibiting a CAGR of 26.42% over the forecast period.
The market is driven by growing investment in immuno-oncology research and development, which supports the advancement of novel and personalized vaccine platforms. Adoption of artificial intelligence and machine learning to enhance vaccine design and neoantigen prediction is improving development efficiency and accelerating clinical success rates.
Key Highlights
- The global peptide cancer vaccine market size was valued at USD 1,850.30 million in 2024.
- The market is projected to grow at a CAGR of 26.42% from 2025 to 2032.
- North America held a market share of 39.55% in 2024, with a valuation of USD 731.79 million.
- The personalized peptide vaccine segment garnered USD 526.41 million in revenue in 2024.
- The breast cancer segment is expected to reach USD 3,016.58 million by 2032.
- Asia Pacific is anticipated to grow at a CAGR of 30.36% over the forecast period.
Major companies operating in the global peptide cancer vaccine market are BioNTech SE, Moderna, Inc., CureVac SE, Immunomic Therapeutics, Inc., Gritstone Bio, Dendreon Pharmaceuticals LLC, Elicio Therapeutics, Imugene Limited, Sellas Life Sciences Group, Inc., ISA Pharmaceuticals B.V., Scancell, Marker Therapeutics, Inc., VAXON Biotech, OncoTherapy Science, Inc., and Lytix Biopharma AS.
Rising global cancer incidence is driving the growth of the peptide cancer vaccine market. In September 2025, the Institute for Health Metrics and Evaluation reported that the global incidence of new cancer cases reached 18.5 million in 2023 and cancer-related deaths rose by 74% to 10.4 million. New cancer cases are projected to increase by 61% over the next 25 years, reaching an estimated 30.5 million by 2050.
Increasing cases of melanoma, lung, prostate, and breast cancers are creating a strong need for effective and personalized immunotherapies. Peptide cancer vaccines are being developed to train the immune system to recognize and destroy tumor-specific antigens.
Growing awareness among healthcare professionals about the benefits of targeted and less toxic cancer treatments is strengthening vaccine adoption. Expanding cancer screening programs are leading to earlier diagnoses, which increases the suitability of patients for peptide-based interventions.
What Factors are Driving Growth in the Market?
Growing investment by pharmaceutical and biotechnology companies in immuno-oncology research and development is strengthening the peptide cancer vaccine market. Pharmaceutical and biotechnology companies are increasingly focusing on peptide-based vaccine platforms due to their ability to target specific tumor antigens with high safety and cost efficiency.
This is driving large-scale funding and strategic collaborations aimed at accelerating vaccine discovery and clinical validation. Moreover, collaboration between academia and industry is supporting the design of personalized vaccines that stimulate stronger immune responses. These coordinated efforts are also expanding the use of peptide vaccines in combination therapies, including checkpoint inhibitors, to improve treatment efficacy.
- In August 2024, Evaxion Biotech announced that it would present one‑year Phase 2 clinical efficacy data for its peptide‑based personalized cancer vaccine EVX‑01, in combination with the checkpoint inhibitor Pembrolizumab, in patients with advanced melanoma.
What Challenges Affect the Production of Personalized Peptide Vaccines?
A key challenge in the peptide cancer vaccine market is the complexity involved in producing personalized formulations based on tumor-specific neoantigens. Developing each vaccine requires tailoring it to an individual’s cancer profile, supported by advanced bioinformatics, precise peptide synthesis, and rigorous quality validation. These processes are time-intensive and significantly increase production costs, limiting scalability and timely treatment availability.
To address this challenge, market players are investing in automated manufacturing platforms, streamlining peptide design workflows, and developing modular production models to reduce cost and turnaround time. These advancements are improving the feasibility and accessibility of personalized peptide vaccines across broader clinical settings.
- In August 2024, NEC Bio Therapeutics and AGC Biologics collaborated to manufacture personalized cancer vaccines. This collaboration supports the development of NECVAX‑NEO1, an orally delivered, bacteria‑based DNA vaccine designed to target patient‑specific tumor neoantigens.
What Role Does AI Play in Vaccine Design Optimization?
A key trend in the peptide cancer vaccine market is the adoption of artificial intelligence and machine learning to enhance vaccine design. These technologies are enabling more accurate prediction of neoantigens that can trigger targeted immune responses. Machine learning algorithms are optimizing peptide sequence selection to improve vaccine efficacy and specificity for individual patients.
Integration of AI is accelerating the preclinical development phase, reducing time and resource requirements for experimental validation. Pharmaceutical and biotech companies are using these insights to streamline clinical trial design and personalize immunotherapy approaches.
- In March 2024, Transgene, NEC Corporation, and BostonGene Corporation expanded their collaboration for the Phase I/II trial of the personalized neoantigen cancer vaccine TG4050. The partnership leverages BostonGene’s molecular and immune profiling capabilities and NEC’s AI‑driven neoantigen prediction to support Transgene’s myvac platform for head and neck cancers.
Peptide Cancer Vaccine Market Report Snapshot
|
Segmentation
|
Details
|
|
By Type
|
Personalized Peptide Vaccine, Peptide-Pulsed Dendritic Cancer Vaccine, Multivalent Peptide Vaccine, Peptide Cocktail Type, Hybrid Peptide Vaccine, Others
|
|
By Application
|
Breast Cancer, Lung Cancer, Prostate Cancer, Melanoma, Others
|
|
By Region
|
North America: U.S., Canada, Mexico
|
|
Europe: France, UK, Spain, Germany, Italy, Russia, Rest of Europe
|
|
Asia-Pacific: China, Japan, India, Australia, ASEAN, South Korea, Rest of Asia-Pacific
|
|
Middle East & Africa: Turkey, U.A.E., Saudi Arabia, South Africa, Rest of Middle East & Africa
|
|
South America: Brazil, Argentina, Rest of South America
|
Market Segmentation
- By Type (Personalized Peptide Vaccine, Peptide-Pulsed Dendritic Cancer Vaccine, Multivalent Peptide Vaccine, Peptide Cocktail Type, Hybrid Peptide Vaccine, and Others): The Personalized Peptide Vaccine segment earned USD 526.41 million in 2024 due to its ability to generate patient-specific immune responses that improve treatment precision and therapeutic efficacy.
- By Application (Breast Cancer, Lung Cancer, Prostate Cancer, Melanoma, and Others): The Breast Cancer segment held 26.82% of the market in 2024, due to the high global incidence of the disease and increasing clinical trials evaluating peptide-based immunotherapies targeting breast tumor antigens.
What Regional Factors are Driving Peptide Vaccine Adoption?
Based on region, the global peptide cancer vaccine market has been classified into North America, Europe, Asia Pacific, Middle East & Africa, and South America.
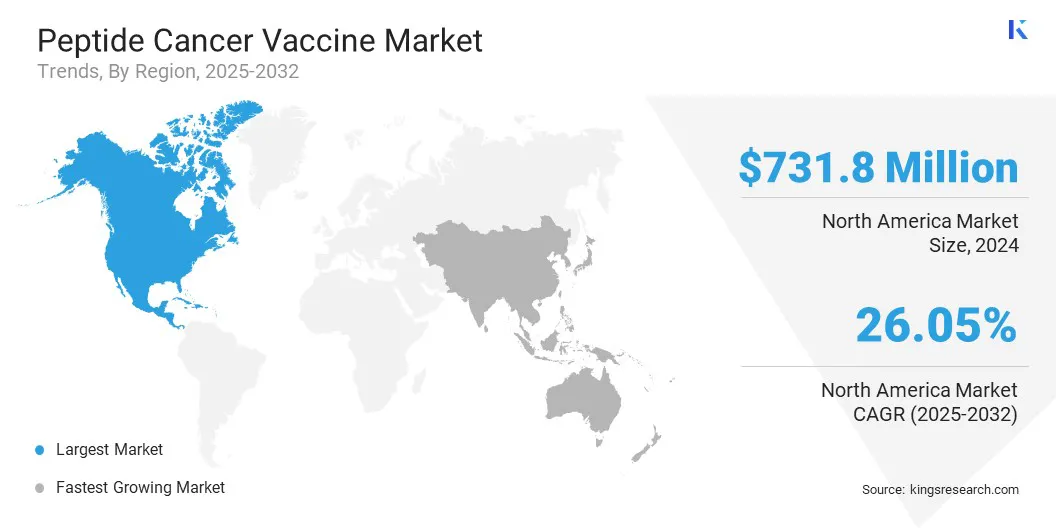
The North America peptide cancer vaccine market share stood around 39.55% in 2024, with a valuation of USD 731.79 million. This dominance is due to advanced laboratories and research facilities focused on immuno-oncology. Cutting-edge technologies such as next-generation sequencing help identify tumor-specific neoantigens efficiently. Research institutions collaborate with biotech companies to translate findings into clinical candidates.
- In March 2025, Icahn School of Medicine at Mount Sinai announced positive results from its Phase 1 trial of vaccine PGV001, a personalized multi‑peptide neoantigen cancer vaccine. The trial included 13 patients with various solid and hematologic malignancies, used computational tumor profiling to select neoantigens, and reported that six of these patients achieved survival at five years, with three of those six being tumor‑
Access to high-quality data supports faster optimization of vaccine formulations. Skilled researchers and technical expertise improve development timelines and reduce errors. Strong infrastructure ensures continuous innovation and strengthens regional market growth.
The market in Asia Pacific is expected to have a significant CAGR of 30.36% over the forecast period. This growth is due to the rising prevalence of cancer cases across the region. In October 2024, the World Health Organization (WHO) South‑East Asia Regional Office reported that the region had an estimated 2.37 million new cancer cases in 2022, and that the cancer burden is projected to increase by approximately 85% by 2050.
Increasing patient numbers create a higher demand for advanced immunotherapies such as peptide vaccines. Early detection programs improve the identification of eligible patients for targeted therapies. Hospitals and clinics integrate these vaccines into personalized treatment plans. The growing patient pool encourages pharmaceutical companies to expand production and research.
- In March 2024, XtalPi Inc. unveiled the progress from its AI‑powered peptide cancer vaccine design platform. The platform integrates an R&D workflow combining dry‑lab AI modelling, automated peptide synthesis, and screening of neoantigen peptides.
Regulatory Frameworks
- In the U.S., the Food and Drug Administration (FDA) treats peptide cancer vaccines as biological products under the Public Health Service Act and the Federal Food, Drug, and Cosmetic Act. Its Center for Biologics Evaluation and Research (CBER) oversees review and licensure via a Biologics License Application. Developers must submit an Investigational New Drug (IND) application, conduct clinical trials, and meet current Good Manufacturing Practice (cGMP) standards (21 CFR Parts 600‑680) for safety, purity, and potency.
- In the European Union (EU), the European Medicines Agency (EMA) applies Regulation (EC) No 1394/2007 on advanced therapy medicinal products (ATMPs) for biologics, including cancer‑immunotherapy vaccines. Member States assess clinical trials, while marketing authorization is through a centralized EMA route. Quality, non‑clinical and clinical data must meet guidelines before approval, and manufacturing must align with GMP.
- In China, the Vaccine Administration Law of the People’s Republic of China governs vaccine development, production, distribution, immunization, and post‑marketing oversight. It mandates full‑life‑cycle quality management, lot release regulations, and risk reporting obligations for vaccine manufacturers. Registration of therapeutic vaccines must align with this law and the National Medical Products Administration (NMPA) standards for biologics.
- In Japan, the Act on Securing Quality, Efficacy, and Safety of Products Including Pharmaceuticals and Medical Devices (PMD Act) regulates biologics and medical devices. The Pharmaceuticals and Medical Devices Agency (PMDA) reviews new drug and biologic applications, ensures manufacturing compliance, and monitors safety. Developers of cancer‑immunotherapy products must meet defined standards for quality, efficacy, and safety and comply with GMP and pharmacovigilance rules.
Competitive Landscape
Major players in the peptide cancer vaccine market are adopting expansion in research and development programs, advancing precision immunotherapy platforms, and forming partnerships with academic and clinical research institutions to remain competitive. Companies are focusing on peptide design technologies that improve immune response against specific tumor mutations.
Investment in early-stage trials and translational studies is increasing to validate therapeutic outcomes and strengthen product pipelines. Firms are also integrating artificial intelligence and bioinformatics to enhance neoantigen identification and vaccine personalization.
- In August 2025, Elicio Therapeutics reported that its peptide vaccine targeting mutant KRAS (mKRAS) achieved a 77% reduction in death risk in a Phase I clinical trial by activating T‑cells against the mutated antigen. This result marks a strong signal of therapeutic benefit for a peptide‑based cancer vaccine in hard‑to‑treat malignancies.
Key Companies in Peptide Cancer Vaccine Market:
- BioNTech SE
- Moderna, Inc.
- CureVac SE
- Immunomic Therapeutics, Inc.
- Gritstone Bio.
- Dendreon Pharmaceuticals LLC
- Elicio Therapeutics
- Imugene Limited
- Sellas Life Sciences Group, Inc.
- ISA Pharmaceuticals B.V.
- Scancell
- Marker Therapeutics, Inc.
- VAXON Biotech
- OncoTherapy Science, Inc.
- Lytix Biopharma ASd
Recent Developments
- In February 2025, IO Biotech published preclinical results for its next‑generation peptide‑vaccine candidate IO112 targeting arginase 1 (Arg1), showing that the vaccine expanded Arg1‑specific T cells and reprogrammed tumor‑associated macrophages. The company plans an Investigational New Drug (IND) submission to the U.S. Food and Drug Administration (FDA) in 2025.
- In April 2024, IO Biotech reported that its off‑the‑shelf therapeutic cancer‑vaccine candidate IO102‑IO103 had new non‑clinical dual‑antigen mechanism data presented at the American Association for Cancer Research Annual Meeting 2024 (AACR).
- In March 2024, XtalPi revealed progress from its AI‑powered peptide R&D platform in designing cancer vaccines, showing that over 80 % of 28 synthesized mutant‑derived peptides demonstrated strong binding to HLA alleles.


