Market Definition
Doxorubicin is an anthracycline chemotherapy agent that inhibits cancer cell growth and replication, offering effective treatment for both hematologic and solid tumors. It is widely used in clinical settings for managing conditions such as bladder cancer, liver cancer, Kaposi sarcoma, leukemia, and lymphoma.
Its role in oncology is expanding through advances in formulation, combination therapies, and targeted delivery approaches that improve efficacy and enhance patient outcomes.
Doxorubicin Market Overview
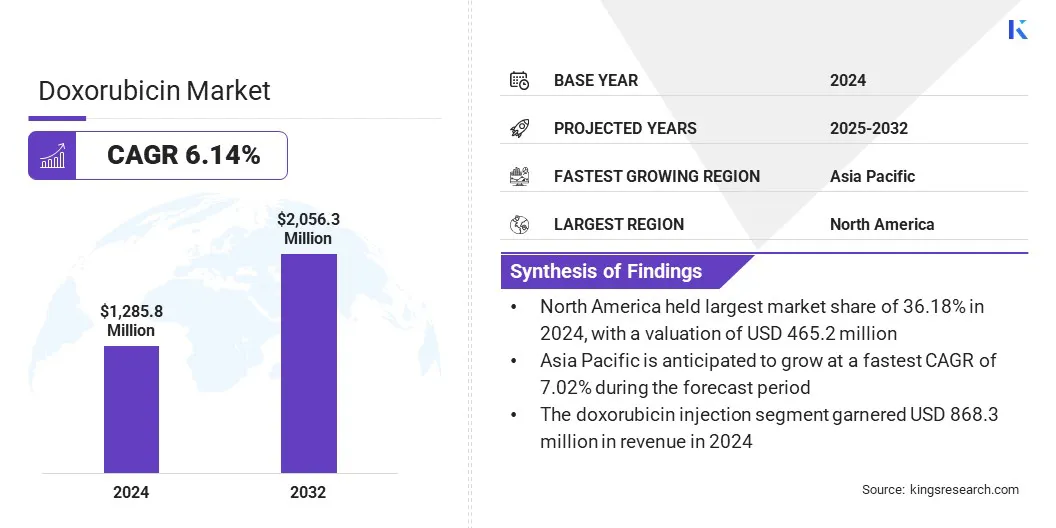
The global doxorubicin market size was valued at USD 1,285.8 million in 2024 and is projected to grow from USD 1,354.7 million in 2025 to USD 2,056.3 million by 2032, exhibiting a CAGR of 6.14% during the forecast period.
This growth is attributed to the rising prevalence of cancer and increasing demand for effective chemotherapy treatments across global healthcare settings. The expansion of hospital networks, oncology centers, and improved awareness of cancer care are further supporting the adoption of doxorubicin.
Key Highlights
- The global doxorubicin market size was USD 1,285.8 million in 2024.
- The market is projected to grow at a CAGR of 6.14% from 2025 to 2032.
- North America held a share of 36.18% in 2024, valued at USD 465.2 million.
- The doxorubicin injection segment garnered USD 868.3 million in revenue in 2024.
- The hospitals pharmacies segment is expected to reach USD 789.2 million by 2032.
- The leukemia segment is anticipated to witness the fastest CAGR of 6.61% over the forecast period.
- Asia Pacific is anticipated to grow at a CAGR of 7.02% through the projection period.
Major companies operating in the global doxorubicin market are Pfizer Inc., Sun Pharmaceutical Industries Ltd., Teva Pharmaceuticals USA, Inc., Baxter, Fresenius Kabi AG, Hikma Pharmaceuticals PLC, Olon S.p.A., Zydus Group, Viatris Inc., Lupin, MicroBiopharm Japan Co., Ltd, Taj Pharmaceuticals Limited, Zhejiang Hisun Pharmaceutical Co. Ltd., Hangzhou Longshine Bio-Tech CO., Ltd, and Dr. Reddy’s Laboratories Ltd.
Increasing focus on advanced formulations, combination therapies, and targeted delivery approaches is promoting wider use of the drug worldwide. Additionally, ongoing clinical research, strategic partnerships, and regulatory support for oncology treatments are boosting market expansion.
- In October 2025, the European Journal of Pharmaceutics and Biopharmaceutics published a study on Talidox (TLD), a liposomal doxorubicin formulation designed to improve drug delivery and reduce systemic toxicity. The research demonstrates that TLD enhances tumor targeting, increases drug stability, and provides controlled release, offering potential improvements in efficacy and safety over conventional doxorubicin.
How is the rising prevalence of cancer worldwide boosting demand for doxorubicin?
The growth of the doxorubicin market is mainly propelled by the rising prevalence of cancer worldwide. According to the World Health Organization (WHO), approximately 20 million new cancer cases and 9.7 million cancer-related deaths were recorded in 2022, with new cases expected to rise by 77% by 2050. Increasing demand for effective chemotherapy treatments for cancers such as leukemia, lymphoma, bladder cancer, and liver cancer is further supporting market expansion.
Expanding hospital networks, specialized oncology centers, and improved healthcare access in emerging regions are enabling wider adoption of doxorubicin. Government initiatives promoting cancer awareness, early detection programs, and funding for oncology treatments are further enhancing accessibility and utilization.
A major challenge impeding the expansion of the doxorubicin market is the drug’s significant side effects, which can limit treatment duration and dosage. Risks such as cardiotoxicity and myelosuppression affect patient compliance and therapeutic outcomes.
Additionally, the development of resistance in cancer cells reduces the drug’s effectiveness over time, creating a need for alternative or combination therapies. High treatment costs in emerging markets further restrict accessibility and adoption, particularly where healthcare infrastructure is limited.
To address these challenges, stakeholders are focusing on advanced drug delivery solutions, including targeted and liposomal formulations, to reduce toxicity and enhance efficacy. Efforts to expand oncology infrastructure, improve patient support programs, and advance research on combination therapies aim to increase accessibility and optimize treatment outcomes. These measures collectively support broader adoption and sustainable growth of the doxorubicin market.
The doxorubicin market is witnessing a growing trend toward the adoption of liposomal formulations to enhance drug delivery, safety, and therapeutic outcomes. Liposomal encapsulation enables targeted release, improved bioavailability, and reduced cardiotoxicity, addressing the limitations of conventional chemotherapy. These advanced formulations are increasingly used for treating ovarian, breast, and multiple myeloma cancers due to their superior efficacy and patient tolerability.
Pharmaceutical manufacturers are expanding research and production capabilities for liposomal variants, supported by rising regulatory approvals and clinical adoption. Continuous innovation in nano carrier design and formulation technology is reinforcing the importance of liposomal doxorubicin in modern cancer therapy.
- In July 2025, the National Center for Biotechnology Information (NCBI) published a review on advances in doxorubicin chemotherapy, emphasizing the use of polymeric nanocarriers to overcome limitations such as cardiotoxicity and poor solubility. The review identified micelles, dendrimers, hydrogels, and polymersomes as effective systems for enhancing stability, targeted delivery, and controlled drug release in cancer therapy.
Doxorubicin Market Report Snapshot
|
Segmentation
|
Details
|
|
By Formulation
|
Lyophilized Powder, and Doxorubicin Injection
|
|
By Distribution
|
Hospitals Pharmacies, Retail Pharmacies, and Online Pharmacies
|
|
By Application
|
Bladder Cancer, Liver Cancer, Kaposi Sarcoma, Leukemia, Lymphoma, Breast Cancer, and Others
|
|
By Region
|
North America: U.S., Canada, Mexico
|
|
Europe: France, UK, Spain, Germany, Italy, Russia, Rest of Europe
|
|
Asia-Pacific: China, Japan, India, Australia, ASEAN, South Korea, Rest of Asia-Pacific
|
|
Middle East & Africa: Turkey, U.A.E., Saudi Arabia, South Africa, Rest of Middle East & Africa
|
|
South America: Brazil, Argentina, Rest of South America
|
Market Segmentation
- By Formulation (Lyophilized Powder and Doxorubicin Injection): The doxorubicin injection segment earned USD 868.3 million in 2024, primarily due to its widespread use in hospitals and oncology centers for treating various cancers.
- By Distribution (Hospitals Pharmacies, Retail Pharmacies, and Online Pharmacies): The hospitals pharmacies held a share of 61.38% in 2024, fueled by their role as primary treatment centers for cancer patients and high demand for chemotherapy administration.
- By Application (Bladder Cancer, Liver Cancer, Kaposi Sarcoma, Leukemia, Lymphoma, Breast Cancer, and Others): The breast cancer segment is projected to reach USD 688.6 million by 2032, owing to the increasing prevalence of breast cancer and growing adoption of doxorubicin-based chemotherapy in treatment protocols.
What is the market scenario in North America and Asia-Pacific region?
Based on region, the global doxorubicin market has been classified into North America, Europe, Asia Pacific, Middle East & Africa, and South America.

North America doxorubicin market share stood at 36.18% in 2024, valued at USD 465.2 million. Favorable healthcare policies, advanced oncology infrastructure, and growing awareness of cancer treatments are reinforcing this expansion. The regional market further benefits from significant investments in cancer research, clinical trials, and hospital networks, which are accelerating the adoption of doxorubicin across multiple cancer types.
Strategic collaborations between pharmaceutical companies, research institutions, and healthcare providers, along with well-established regulatory frameworks, further strengthen growth prospects. Continuous improvements in drug delivery systems, patient support programs, and treatment protocols further position North America as a key hub for doxorubicin utilization and innovation.
Asia Pacific doxorubicin market is set to grow at a CAGR of 7.02% over the forecast period. This growth is fueled by rising cancer prevalence, expanding healthcare infrastructure, and increasing access to oncology treatments across the region. Government initiatives, healthcare funding, and awareness programs are strengthening the oncology care ecosystemin Asia Pacific.
Increasing adoption of advanced formulations, combination therapies, and hospital-based chemotherapy programs is presenting potential opportunities for improved patient outcomes. Additionally, ongoing investments by pharmaceutical companies, expansion of specialty oncology centers, and advancements in drug delivery systems are leading to the widespread adoption of doxorubicin across Asia Pacific.
- In June 2025, Alembic Pharmaceuticals received final FDA approval for its ANDA of doxorubicin hydrochloride liposome injection, available in 20 mg/10 mL and 50 mg/25 mL single-dose vials. The generic formulation, equivalent to Baxter’s Doxil, is indicated for ovarian cancer, AIDS-related Kaposi’s sarcoma, and multiple myeloma.
Regulatory Frameworks
- In the U.S., the U.S. Food and Drug Administration (FDA) regulates chemotherapeutic drugs, including doxorubicin. It ensures the safety, efficacy, labeling, and post-market surveillance of doxorubicin products, maintaining high standards for patient treatment and clinical use.
- In the EU, the European Medicines Agency (EMA) governs anticancer medications, including doxorubicin. It oversees centralized marketing authorization, pharmacovigilance, and quality control, ensuring that doxorubicin products meet rigorous safety and efficacy requirements across member states.
- In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) oversees oncology drugs, including doxorubicin. It provides guidelines for clinical trials, approval, and risk management to safeguard patient health and support safe clinical administration.
- In India, the Central Drugs Standard Control Organization (CDSCO) regulates chemotherapeutic agents, including doxorubicin. It monitors approval processes, quality standards, and post-market surveillance to ensure safe and effective use in hospitals and oncology centers.
Competitive Landscape
Companies operating in the global doxorubicin market are maintaining competitiveness through investments in advanced drug formulations, targeted delivery systems, and combination therapy development. They are focusing on expanding production capacity, enhancing manufacturing efficiency, and ensuring consistent supply to meet growing demand across hospitals, oncology centers, and emerging markets.
Key players are broadening their portfolios to include liposomal doxorubicin, generics, and novel chemotherapeutic combinations, supported by strategic collaborations, licensing agreements, and partnerships with healthcare providers.
Moreover, there is a growing focus on enhancing collaboration with research institutions, healthcare providers, and regulatory authorities to accelerate clinical trial execution and streamline the approval process. Additionally, companies are improving patient support programs, treatment monitoring, and integrated healthcare services, while leveraging digital tools and innovative distribution networks to maintain a competitive edge.
Top Key Companies in Doxorubicin Market:
- Pfizer Inc.
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceuticals USA, Inc.
- Baxter
- Fresenius Kabi AG
- Hikma Pharmaceuticals PLC
- Olon S.p.A.
- Zydus Group
- Viatris Inc.
- Lupin
- MicroBiopharm Japan Co., Ltd
- Taj Pharmaceuticals Limited
- Zhejiang Hisun Pharmaceutical Co. Ltd.
- Hangzhou Longshine Bio-Tech CO., Ltd
- Reddy’s Laboratories Ltd.
Recent Developments (Product Launch)
- In August 2024, Lupin launched doxorubicin hydrochloride liposome injection in the U.S. after receiving FDA approval for its Abbreviated New Drug Application (ANDA) through ForDoz Pharma. This generic version of Doxil is indicated for treating ovarian cancer, AIDS-related Kaposi’s sarcoma, and multiple myeloma.


