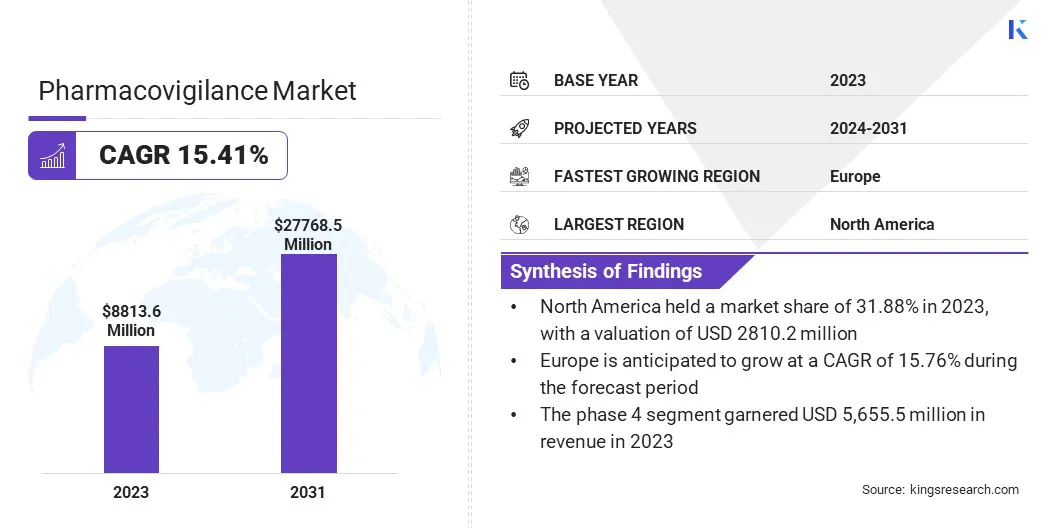
Pharmacovigilance Market Size
The global Pharmacovigilance Market size was valued at USD 8,813.6 million in 2023 and is projected to reach USD 27,768.5 million by 2031, growing at a CAGR of 15.41% from 2024 to 2031. In the scope of work, the report includes solutions offered by companies such as IQVIA Inc, Labcorp, ICON plc, Cognizant Technology Solutions Corporation, PAREXEL International Corporation, IBM Watson Health, Accenture plc, Capgemini SE, ArisGlobal LLC, Oracle Corporation and Others. The market is experiencing significant growth due to the increasing number of drug approvals and the expansion of industries worldwide.
As pharmaceutical companies develop and introduce new drugs to the market, there is a pressing need for robust pharmacovigilance systems to monitor the safety and efficacy of these products. This trend is particularly evident in emerging economies, where improvements in healthcare infrastructure are leading to higher demand for pharmaceutical products.
Moreover, advancements in medical research and technologies are accelerating the drug development process, resulting in an increased number of products entering clinical trials and obtaining regulatory approvals. This surge in drug approvals contributes to the expansion of the pharmaceutical market and stimulates the need for pharmacovigilance services to ensure post-market surveillance and regulatory compliance across different regions.
Pharmacovigilance encompasses the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. Its application areas include monitoring the safety of pharmaceutical products, vaccines, medical devices, and herbal remedies post-approval. With the evolving technical landscape, pharmacovigilance integrates advanced technologies such as artificial intelligence (AI), machine learning (ML), natural language processing (NLP), and big data analytics to enhance adverse event detection and signal prioritization.
- Regulatory bodies such as the FDA and EMA continually update pharmacovigilance guidelines, emphasizing the need for proactive risk management, signal detection, and real-time safety monitoring throughout a product lifecycle.
Analyst’s Review
Key players in the pharmacovigilance market are implementing strategic approaches such as investing in advanced technologies for efficient data management and signal detection, fostering collaborations between pharmaceutical companies and regulatory agencies to ensure compliance and timely reporting, utilizing outsourcing partnerships for cost-effective and specialized expertise, and prioritizing continuous training and skill development for pharmacovigilance professionals.
These strategies address current market demands and position companies for future growth by adapting to evolving regulatory landscapes, emerging market trends, and technological innovations that shape the pharmacovigilance industry.
Pharmacovigilance Market Growth Factors
The growing focus on patient safety and regulatory compliance is a key factor driving the growth of the pharmacovigilance market. Pharmaceutical companies and regulatory agencies are increasingly prioritizing patient-centric approaches to drug development and monitoring. This includes stringent safety monitoring throughout clinical trials (pre-market), as well as continuous surveillance post-market to detect and mitigate adverse events promptly.
Regulatory frameworks such as Good Pharmacovigilance Practices (GVP) and international standards such as ICH E2E guidelines mandate comprehensive pharmacovigilance strategies to ensure drug safety, public health protection, and regulatory compliance across global markets. This emphasis on patient safety and regulatory adherence underscores the critical role of pharmacovigilance in maintaining public trust and ensuring the effectiveness of healthcare interventions.
However, data privacy concerns and security risks associated with handling sensitive patient information and adverse event data pose significant challenges to market growth. To address these challenges, pharmacovigilance stakeholders are implementing robust data encryption protocols, access controls, and secure data storage solutions.
Compliance with data protection regulations, such as GDPR in Europe and HIPAA in the United States, is crucial for safeguarding patient privacy and maintaining data integrity. Investing in cybersecurity measures, conducting regular audits, and fostering a culture of data security awareness among employees are essential steps to overcome data privacy concerns in pharmacovigilance operations.
Pharmacovigilance Market Trends
The increased focus on post-market surveillance and signal detection, coupled with the widespread adoption of cloud-based pharmacovigilance solutions, is fostering the growth of the pharmacovigilance market. Post-market surveillance plays a pivotal role in continuously monitoring the safety profile of drugs and medical products after they enter the market.
Moreover, advanced signal detection methodologies powered by AI, ML, and big data analytics enable pharmacovigilance teams to identify potential safety signals early, prioritize risks, and implement targeted risk mitigation strategies. The adoption of cloud-based pharmacovigilance platforms offers scalability, real-time data access, and enhanced collaboration among global pharmacovigilance teams, supporting efficient adverse event reporting and ensuring regulatory compliance. These trends are anticipated to fuel the expansion of the market in the coming years, fostering innovation and improving patient safety outcomes.
Segmentation Analysis
The global market is segmented based on clinical trial phase, service provider, end-use, and geography.
By Clinical Trial Phase
Based on the clinical trial phase, the pharmacovigilance market is segmented into pre-clinical, phase 1, phase 2, phase 3, and phase 4. The phase 4 segment garnered the highest revenue of USD 5,655.5 million in 2023. The increasing emphasis on post-market surveillance and pharmacovigilance activities after drug approval is propelling the growth of the segment. Phase 4 trials, also known as post-marketing surveillance trials, are critical for monitoring the safety and efficacy of drugs over an extended period. These trials provide valuable insights into long-term safety profiles, rare adverse events, drug interactions, and effectiveness in diverse patient groups.
Pharmaceutical companies and regulatory agencies are prioritizing Phase 4 studies to fulfill post-market safety commitments, comply with regulatory requirements, and ensure ongoing risk-benefit assessments for marketed drugs, thereby contributing significantly to the prominence of this segment in the market.
By Service Provider
Based on service provider, the market is bifurcated into in-house and contract outsourcing. The contract outsourcing segment held the largest pharmacovigilance industry share of 68.73% in 2023 due to the increasing trend among pharmaceutical companies to outsource pharmacovigilance activities to specialized service providers and contract research organizations (CROs). Outsourcing offers cost-effective solutions, access to skilled personnel, scalability, and flexibility in pharmacovigilance operations, allowing companies to focus on core drug development activities.
Additionally, outsourcing partnerships enable access to global regulatory expertise, compliance with evolving pharmacovigilance regulations, and fostering efficient adverse event reporting and management, thus driving the growth of the contract outsourcing segment in the pharmacovigilance market.
By End-Use
Based on End-Use, the market is categorized into pharmaceutical & biotechnology industry, medical device companies, and others. The medical device companies segment is anticipated to witness significant growth at a CAGR of 17.17% over the forecast period due to the increasing adoption of medical devices globally. Pharmacovigilance practices are extending beyond pharmaceuticals to encompass medical devices, requiring robust post-market surveillance, adverse event reporting, and risk management strategies specific to device-related incidents.
As the medical device industry is innovating and introducing new technologies, the demand for pharmacovigilance services tailored to medical devices is expected to surge, driving growth in the medical device companies segment.
Pharmacovigilance Market Regional Analysis
Based on region, the global market is classified into North America, Europe, Asia-Pacific, MEA, and Latin America.
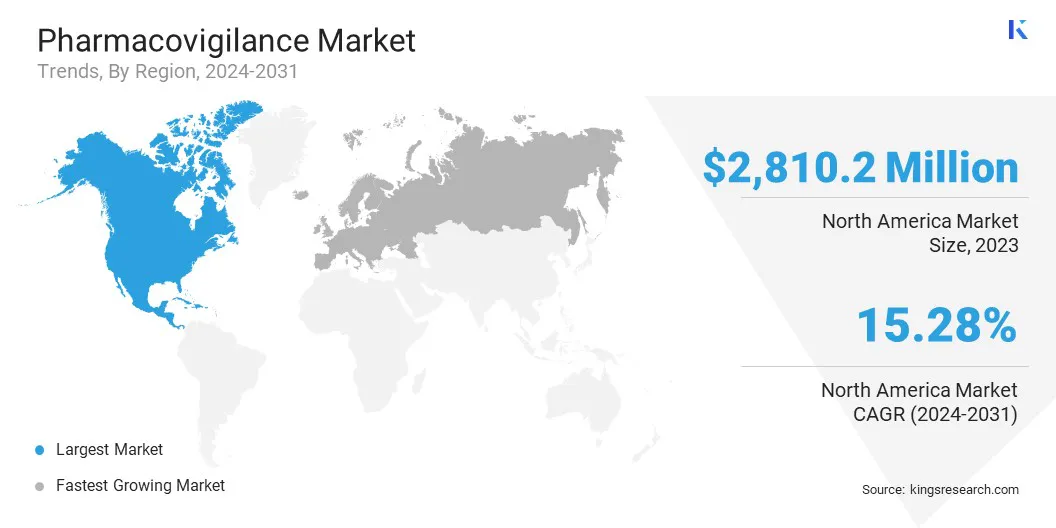
The North America Pharmacovigilance Market share stood around 31.88% in 2023 in the global market, with a valuation of USD 2810.2 million, primarily due to its well-established healthcare infrastructure, stringent regulatory standards, robust pharmacovigilance practices, and significant investments in drug development and safety monitoring. The region houses key pharmaceutical companies, leading research institutions, and regulatory agencies such as the FDA, which is contributing significantly to the pharmacovigilance ecosystem.
- Moreover, increased drug approvals, growing concerns about drug safety, and advancements in pharmacovigilance technologies bolster the region’s position in the market.
Europe is poised to witness notable growth over the forecast period at a CAGR of 15.76% owing to a well-established pharmaceutical industry, which includes leading players in drug development. This infrastructure is facilitating robust pharmacovigilance practices and regulatory compliance, thereby driving regional market expansion. Moreover, European regulatory bodies such as the European Medicines Agency (EMA) are playing a pivotal role in setting pharmacovigilance guidelines and ensuring drug safety standards across member states.
The implementation of stringent pharmacovigilance regulations such as the EU Pharmacovigilance System and the Pharmacovigilance Risk Assessment Committee (PRAC) underscores Europe's commitment to public health protection and pharmacovigilance excellence. Additionally, the region's increasing focus on real-world evidence (RWE) generation, adoption of digital health technologies, and collaborations between industry stakeholders and research institutions are contributing to a dynamic pharmacovigilance landscape in Europe.
Competitive Landscape
The pharmacovigilance market report will provide valuable insight with an emphasis on the fragmented nature of the industry. Prominent players are focusing on several key business strategies such as partnerships, mergers and acquisitions, product innovations, and joint ventures to expand their product portfolio and increase their market shares across different regions. Companies are undertaking effective strategic initiatives, including investments in R&D activities, the establishment of new manufacturing facilities, and supply chain optimization, to gain a competitive edge in the market.
List of Key Companies in Pharmacovigilance Market
- IQVIA Inc
- Labcorp (Laboratory Corporation of America Holdings)
- ICON plc
- Cognizant Technology Solutions Corporation
- PAREXEL International Corporation
- IBM Watson Health
- Accenture plc
- Capgemini SE
- ArisGlobal LLC
- Oracle Corporation
Key Industry Developments
- August 2023 (Partnership): Deloitte partnered with IviGee, a leading provider of pharmacovigilance solutions. This collaboration was aimed to enhance pharmacovigilance services for pharmaceutical companies by combining Deloitte's expertise in life sciences with IviGee's innovative technology platform. The partnership was anticipated to enable more efficient adverse event monitoring, regulatory compliance, and risk management, thus improving patient safety and product quality in the pharmaceutical industry.
- April 2023 (Partnership): Pharmalex, a global provider of development consulting and scientific services for the pharmaceutical, biotech, and medical device industries, expanded its footprint in the Asia-Pacific by acquiring cPharm Australia. This strategic move enhanced Pharmalex's pharmacovigilance capabilities in the region and enabled the company to offer comprehensive regulatory and safety services to its clients in Australia and across the Asia-Pacific region.
The global Pharmacovigilance Market is segmented as:
By Clinical Trial Phase
- Pre-clinical
- Phase 1
- Phase 2
- Phase 3
- Phase 4
By Service Provider
- In-House
- Contract Outsourcing
By End-Use
- Pharmaceutical & Biotechnology Industry
- Medical Device Companies
- Others
By Region
- North America
- Europe
- France
- U.K.
- Spain
- Germany
- Italy
- Russia
- Rest of Europe
- Asia-Pacific
- China
- Japan
- India
- South Korea
- Rest of Asia-Pacific
- Middle East & Africa
- GCC
- North Africa
- South Africa
- Rest of Middle East & Africa
- Latin America
- Brazil
- Argentina
- Rest of Latin America


