Migraine Drugs Market Overview
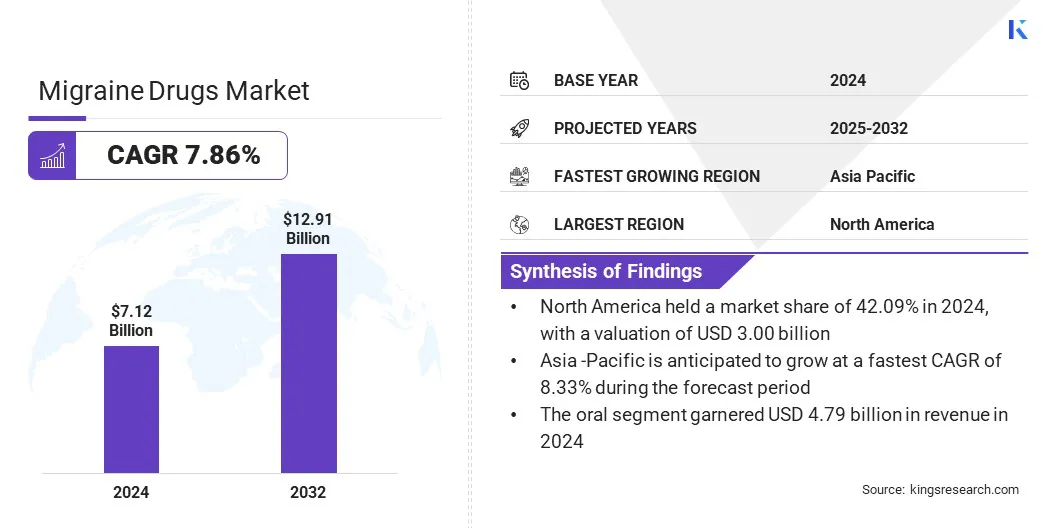
The global migraine drugs market size was valued at USD 7.12 billion in 2024 and is projected to grow from USD 7.60 billion in 2025 to USD 12.91 billion by 2032, exhibiting a CAGR of 7.86% during the forecast period. The market growth is driven by the rising prevalence of migraine and growing awareness of advanced treatment options focused on rapid relief and better clinical outcomes.
Continuous innovation in drug delivery technologies and regulatory support for non-oral therapies are further enhancing treatment accessibility and expanding market opportunities.
Key Market Highlights:
- The global market size was recorded at USD 7.12 billion in 2024.
- The market is projected to grow at a CAGR of 7.86% from 2025 to 2032.
- North America held a market share of 42.09% in 2024, with a valuation of USD 3.00 billion.
- The triptans segment garnered USD 2.48 billion in revenue in 2024.
- The oral segment is expected to reach USD 8.48 billion by 2032.
- The preventive treatment segment is anticipated to grow at a CAGR of 8.02% over the forecast period.
- The hospital pharmacies segment held a market share of 44.12% in 2024.
- Asia Pacific is anticipated to grow at a CAGR of 8.33% through the projection period.
Major companies operating in the migraine drugs industry are AbbVie Inc., Amgen Inc., Teva Pharmaceutical Industries Ltd, Eli Lilly and Company, Pfizer Inc., Bausch Health Companies Inc., Gensco Pharma, Impel Pharmaceuticals LLC, Tonix Pharmaceuticals Holding Corp, H. Lundbeck A/S, Amgen Inc and Novartis Pharmaceuticals Corporation.
Migraine Drugs Market Overview
The migraine drugs market is driven by the development of innovative and patient-centric treatment delivery systems that enable faster and more convenient access to therapy. Autoinjectors and non-oral formulations are reducing reliance on hospital visits by allowing self-administration during acute attacks.
These advancements are improving patient adherence and addressing gaps in treatment access, thereby contributing to market growth.
- In May 2025, Amneal Pharmaceuticals received the U.S. Food and Drug Administration (FDA) approval for Brekiya a autoinjector for the acute treatment of migraine with or without aura and cluster headaches in adults, making it the first and only ready-to-use DHE autoinjector for self-administration.
Market Driver
Advancements in drug development
Advancements in drug development are driving the market by enhancing the effectiveness, safety, and consistency of migraine treatments. These improvements are leading to better patient outcomes and strengthening physician confidence in recommending newer therapies.
With increasing research in drug development the market is witnessing a steady rise in approved medications that offer greater reliability and longer lasting relief for patients. This is encouraging wider adoption across healthcare systems and supporting the continued growth of the market.
- In January 2025, the U.S. Food and Drug Administration (FDA) approved SYMBRAVO (AXS-07) for the acute treatment of migraine, reflecting advancements in drug development aimed at delivering faster and longer-lasting relief. Clinical data demonstrating pain freedom within two hours and sustained effects up to 48 hours support its potential to improve treatment outcomes and patient satisfaction.
Market Challenge
High cost of novel therapies
The high cost of novel therapies such as CGRP inhibitors and biologic agents is posing a significant challenge to the growth of the migraine drugs market.
These treatments offer improved clinical outcomes and consistent safety profiles. However, the high cost limits accessibility for a large segment of patients across different healthcare systems.This restricts broader adoption and affects treatment continuity, particularly in low- and middle-income regions.
To address this challenge, market players are increasingly focusing on implementing strategic pricing models by expanding patient assistance programs and collaborating with healthcare providers and insurers to improve affordability and access.
Additionally, companies are investing in the development of cost-effective biosimilar and next-generation therapies that retain clinical effectiveness while reducing manufacturing expenses. These efforts aim to ease financial barriers and promote the broader adoption of advanced migraine treatments across diverse patient populations.
Market Trend
Increased R&D investment in therapies and next-generation drug delivery systems
Increased research and development investment in combination therapies and next-generation drug delivery systems is significantly shaping the market.
Companies are focusing on developing treatments that provide faster symptom relief and improved clinical outcomes by addressing the limitations of conventional oral medications.
These advancements are enhancing patient convenience, improving therapeutic efficacy and enabling more personalized treatment approaches. The shift toward innovative delivery methods is supporting the wider adoption and access across varied patient populations and healthcare settings.
- In September 2024, Tonix Pharmaceuticals received U.S. Patent No. 12,097,183 for the subcutaneous delivery of the Zembrace SymTouch composition for the acute treatment of migraine in adults. The patent supports the FDA-approved autoinjector by providing market exclusivity until 2036.
Migraine Drugs Market Report Snapshot
|
Segmentation
|
Details
|
|
By Drug Class
|
Triptans, CGRP Monoclonal Antibodies, Gepants (CGRP receptor antagonists), Ergot Alkaloids, NSAIDs, Others
|
|
By Route of Administration
|
Oral, Injectable, Nasal
|
|
By Treatment Type
|
Acute Treatment, Preventive Treatment
|
|
By Distribution Channel
|
Hospital Pharmacies, Retail Pharmacies, Online Pharmacies
|
|
By Region
|
North America: U.S., Canada, Mexico
|
|
Europe: France, UK, Spain, Germany, Italy, Russia, Rest of Europe
|
|
Asia-Pacific: China, Japan, India, Australia, ASEAN, South Korea, Rest of Asia-Pacific
|
|
Middle East & Africa: Turkey, U.A.E., Saudi Arabia, South Africa, Rest of Middle East & Africa
|
|
South America: Brazil, Argentina, Rest of South America
|
Market Segmentation:
- By Drug Class (Triptans, CGRP Monoclonal Antibodies, Gepants (CGRP receptor antagonists), Ergot Alkaloids, NSAIDs and Others): The triptans segment earned USD 2.48 billion in 2024 due to their established efficacy, rapid onset of action, and wide availability for acute migraine relief.
- By Route of Administration (Oral, Injectable and Nasal): The oral segment held 67.23% of the market in 2024, due to ease of administration and broad prescription coverage.
- By Treatment Type (Acute Treatment and Preventive Treatment): The acute treatment segment is projected to reach USD 7.99 billion by 2032, owing to the high prevalence of episodic migraine attacks and the need for fast-acting symptom relief.
- By Distribution Channel (Hospital Pharmacies, Retail Pharmacies and Online Pharmacies,): The retail pharmacies segment is anticipated to grow at a CAGR of 9.01% over the forecast period, due to increased consumer access and expanding over-the-counter migraine options.
Migraine Drugs Market Regional Analysis
Based on region, the global market has been classified into North America, Europe, Asia Pacific, Middle East & Africa, and South America.
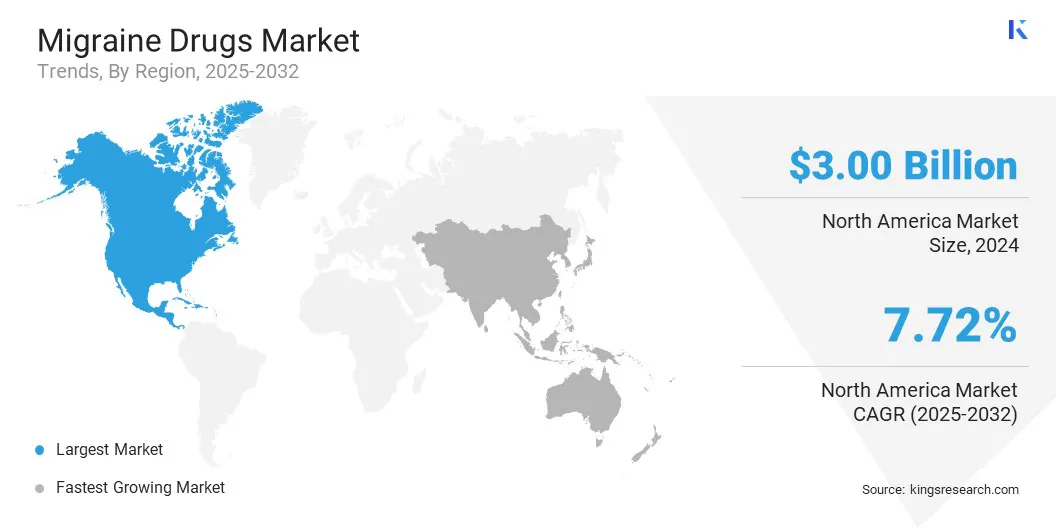
North America migraine drugs market accounted for a share of around 42.09% in 2024, with a valuation of USD 3.00 billion. This dominance is attributed to the presence of major pharmaceutical companies actively introducing advanced drug delivery technologies.
These innovations are driving market growth by improving patient adherence and therapeutic outcomes. Additionally, companies in the region are focusing on user-friendly formulations that simplify administration, such as nasal sprays and auto-injectors, enhancing treatment accessibility and reducing dependence on traditional oral tablets.
Additionally, the market is benefiting from favorable regulatory support and rapid approvals that enable faster commercialization of new therapies.
Companies in this region are actively collaborating with healthcare providers to improve affordability and expand treatment coverage, allowing a broader population to access effective migraine care. These factors are driving the growth of the North America market.
- In May 2025, Gensco Pharma acquired the global rights and intellectual property for RizaFilm and RizaPort, rizatriptan-based oral dissolvable film formulations for acute migraine treatment. The company aims to improve access and affordability through region-specific pricing strategies and convenient, fast-acting, water-free administration.
The Asia Pacific migraine drugs industry is set to grow at a CAGR of 8.33% over the forecast period. This growth is attributed to the rising regulatory approvals and technological adoption that are transforming the market across the region.
The approval of home-based neuromodulation devices is allowing the market to expand beyond traditional drug therapies, particularly in countries with advanced digital health infrastructure.
Additionally, growing demand for non-oral treatment options, driven by the prevalence of conditions like gastroparesis is prompting countries such as Japan and others in Asia Pacific to adopt these innovative solutions, thereby contributing to the market expansion.
- In January 2024, Neurolief received approval from Japan's Ministry of Health, Labor and Welfare (MHLW) for Relivion, a non-invasive neuromodulation device, making it the first at-home neuromodulation treatment for acute migraine in the country. This approval allows Neurolief and its partner Sawai Pharmaceutical Co. to commercialize Relivion in Japan, offering patients an alternative to conventional drug-based therapies.
Regulatory Frameworks
- In the U.S., Food and Drug Administration (FDA) regulates the market by overseeing drug approvals, clinical trial protocols, manufacturing standards, labeling, marketing, and post-market surveillance. The FDA ensures all migraine medications meet strict safety, efficacy, and quality standards before they reach patients, while also monitoring adverse events and compliance.
- In China, the National Medical Products Administration (NMPA) governs the regulation of migraine drugs by overseeing the drug registration process, clinical trial approvals, quality control, safety monitoring, and market authorization. The NMPA plays a central role in ensuring that all migraine medications meet national standards for efficacy, safety, and production practices.
- In India, the Central Drugs Standard Control Organization (CDSCO) regulates the market by supervising drug approvals, clinical trials, manufacturing licenses, and pharmacovigilance. It ensures that all migraine medications meet prescribed quality, safety, and efficacy standards while also enforcing compliance with national drug control policies and regulatory frameworks.
- In the UK, the Medicines and Healthcare Products Regulatory Agency (MHRA) is responsible for regulating migraine drugs by overseeing clinical trials, product licensing, manufacturing standards, advertising, and post-market monitoring. MHRA ensures that migraine treatments comply with UK legislation regarding drug safety, effectiveness, quality control, and pharmacovigilance obligations.
Competitive Landscape
Major players in the migraine drugs market are launching integrated digital platforms through strategic partnerships with telehealth providers, pharmacies and symptom tracking apps to enhance patient engagement and treatment outcomes.
They are launching integrated digital platforms through collaborations with telehealth providers, digital pharmacies, and symptom tracking app developers.
Additionally, market players are focusing on differentiating their offerings by launching integrated digital platforms and strengthening brand presence to maintain relevance and competitiveness in the market.
- In April 2025, Tonix Pharmaceuticals partnered with UpScript, Blink Health, and a migraine diary app to launch TONIX ONE, a fully-integrated digital platform aimed at improving migraine management. The platform connects patients with telehealth services, e-prescriptions, and real-time symptom tracking, streamlining access to non-oral therapies and promoting education on migraine care, particularly for patients affected by gastroparesis.
Key Companies in Migraine Drugs Market:
- AbbVie Inc
- Amgen Inc
- Teva Pharmaceutical Industries Ltd.
- Eli Lilly and Company
- Pfizer Inc
- Bausch Health Companies Inc
- Gensco Pharma
- Impel Pharmaceuticals LLC
- Tonix Pharmaceuticals Holding Corp.
- Lundbeck A/S
- Amgen Inc
- Novartis Pharmaceuticals Corporation
Recent Developments (New Product Launch)
- In April 2025, Teva Pharmaceuticals received a supplemental Biologics License Application (sBLA) approval from the U.S. Food and Drug Administration (FDA) for AJOVY (fremanezumab), expanding its use for the prevention of episodic migraine in pediatric patients aged 6 to 17 years. This approval supports the broader clinical application of the therapy across younger patient populations.
- In April 2025, Satsuma Pharmaceuticals received FDA approval for Atzumi nasal powder, intended for the acute treatment of migraine in adults. The formulation offers a convenient, needle-free alternative to traditional migraine therapies, designed for rapid absorption and on-the-go use.
the


