Market Definition
Chemiluminescence immunoassay (CLIA) is an advanced diagnostic technique that combines immunochemical reactions with chemiluminescence detection to quantify analytes such as proteins, hormones, and infectious agents in biological samples.
It offers high sensitivity, specificity, and rapid turnaround times compared to traditional immunoassays. Widely used in clinical diagnostics, including infectious diseases, endocrinology, oncology, and cardiology, CLIA has become a vital tool in modern laboratory medicine.
Chemiluminescence Immunoassay Market Overview
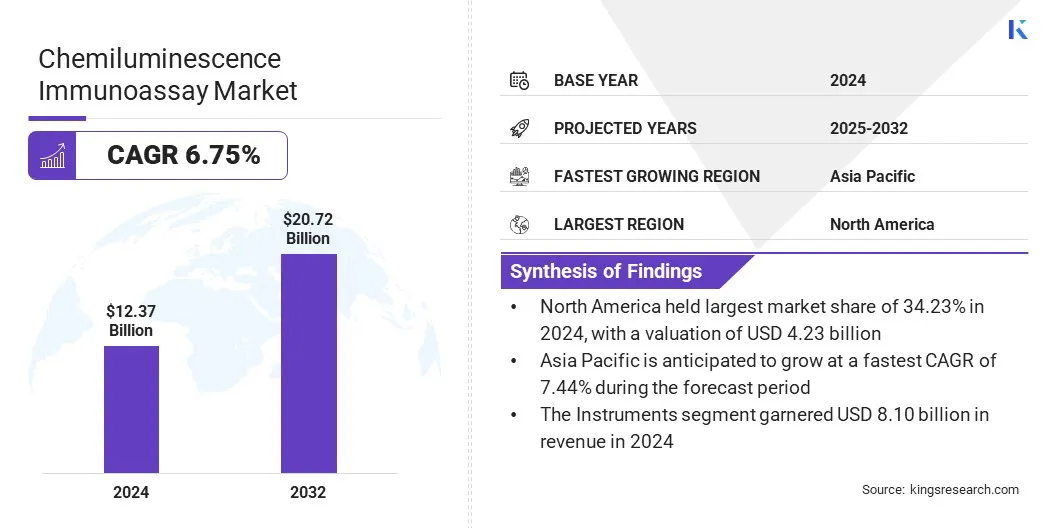
The global chemiluminescence immunoassay market size was valued at USD 12.37 billion in 2024 and is projected to grow from USD 13.11 billion in 2025 to USD 20.72 billion by 2032, exhibiting a CAGR of 6.75% during the forecast period.
The market is experiencing steady growth, driven by rising demand for accurate and rapid diagnostic solutions. The increasing prevalence of chronic and infectious diseases, coupled with advancements in automation and high-throughput systems, is further supporting this expansion across clinical laboratories, hospitals, and research settings.
Key Highlights:
- The chemiluminescence immunoassay industry size was recorded at USD 12.37 billion in 2024.
- The market is projected to grow at a CAGR of 6.75% from 2024 to 2032.
- North America held a share of 6.82% in 2024, valued at USD 4.23 billion.
- The instruments segment garnered USD 8.10 billion in revenue in 2024.
- The electrochemiluminescence segment is expected to reach USD 12.74 billion by 2032.
- The infectious diseases segment is projected to register a share of 36.34% by 2032.
- The hospitals & clinics segment accounted for a share of 42.12% in 2024.
- Asia Pacific is anticipated to grow at a CAGR of 7.44% over the forecast period.
Major companies operating in the chemiluminescence immunoassay market are F. Hoffmann-La Roche Ltd, Siemens Medical Solutions USA, Inc., DH Life Sciences, LLC., Autobio, DiaSorin S.p.A., Mindray Medical India Pvt. Ltd., Beckman Coulter, Inc., Ozelle, Nanjing Poclight Biotechnology Co., Ltd., Biovantion Inc., Zecen Biotech CO., LTD., Sysmex Corporation, Shenzhen New Industries Biomedical Engineering Co., Ltd., Diatron, Getein Biotech, Inc., and others.
Market growth is fueled by technological innovations and strategic initiatives from leading manufacturers. Numerous players are introducing advanced platforms with enhanced automation and multiplexing capabilities to meet growing demand for high-throughput testing.
- For instance, in April 2025, Beckman Coulter launched its DxI 9000 Access Immunoassay Analyzer, designed to deliver faster turnaround and improved workflow efficiency in clinical laboratories.
Such innovations are strengthening diagnostic capacity while supporting healthcare systems in addressing rising testing volumes, improving operational efficiency, and achieving better clinical outcomes.
Market Driver
Rising Prevalence of Chronic and Infectious Diseases
The expansion of the chemiluminescence immunoassay (CLIA) market is primarily fueled by the increasing prevalence of chronic and infectious diseases, which continues to place significant pressure on global healthcare systems.
- According to the World Health Organization (WHO), non-communicable diseases such as cardiovascular disorders, diabetes, cancer, and chronic respiratory conditions cause nearly 75% of global deaths, exceeding 40 million annually.
This growing disease burden is creating demand for advanced diagnostic technologies capable of delivering accurate, sensitive, and rapid test results. CLIA systems, with their ability to detect low concentrations of biomarkers and provide higher specificity compared to conventional immunoassays, have emerged as a preferred diagnostic tool in clinical settings, propelling market expansion.
Market Challenge
Cost and Operational Barriers
A key challenge in the chemiluminescence immunoassay market is the high cost associated with advanced instruments and consumables, which limits adoption in resource-constrained healthcare settings.
The significant investment required for automated CLIA analyzers and specialized reagents places a significant financial burden on smaller hospitals, diagnostic laboratories, and facilities in developing regions. These cost barriers reduce accessibility and widen the diagnostic gap between developed and emerging markets.
To address this challenge, market players are developing cost-efficient systems and reagent rental models that reduce upfront expenditure for laboratories. Additionally, they are increasingly developing scalable CLIA platforms that led laboratories to expand testing capacity in line with patient demand.
Market Trend
Surging Adoption of Automation and AI
A notable trend influencing the chemiluminescence immunoassay market is the integration of automation and artificial intelligence (AI) to enhance diagnostic efficiency and accuracy. Automated CLIA systems reduce manual intervention, minimize error rates, and enable high-throughput testing in clinical laboratories.
AI-powered algorithms are increasingly being applied to optimize assay interpretation, support predictive diagnostics, and facilitate early disease detection. These advancements improve clinical decision-making and allow laboratories to manage growing patient volumes with improved cost-efficiency and consistent diagnostic quality, thereby contributing to market growth.
- For instance, in July 2023, Siemens Healthineers introduced the Atellica CI Analyzer, a fully automated platform integrating AI and automation. The product is designed to address staffing shortages, increase productivity, and enhanced laboratory efficiency and reliability.
Chemiluminescence Immunoassay Market Report Snapshot
|
Segmentation
|
Details
|
|
By Product
|
Instruments, Consumables, Software & Services
|
|
By Technology
|
Chemiluminescence Enzyme Immunoassay, Electrochemiluminescence, Microparticle Chemiluminescence
|
|
By Application
|
Infectious Diseases, Oncology, Endocrinology, Cardiology, Others
|
|
By End-User
|
Hospitals & Clinics, Diagnostic Laboratories, Research & Academic Institutes, Pharmaceutical & Biotechnology Companies
|
|
By Region
|
North America: U.S., Canada, Mexico
|
|
Europe: France, UK, Spain, Germany, Italy, Russia, Rest of Europe
|
|
Asia-Pacific: China, Japan, India, Australia, ASEAN, South Korea, Rest of Asia-Pacific
|
|
Middle East & Africa: Turkey, U.A.E., Saudi Arabia, South Africa, Rest of Middle East & Africa
|
|
South America: Brazil, Argentina, Rest of South America
|
Market Segmentation
- By Product (Instruments, Consumables, and Software & Services): The instruments segment earned USD 8.10 billion in 2024, mainly due to the growing adoption of automated platforms in hospitals and laboratories.
- By Technology (Chemiluminescence Enzyme Immunoassay, Electrochemiluminescence, and Microparticle Chemiluminescence): The Chemiluminescence Enzyme Immunoassay held a share of 45.32% in 2024, supported by its widespread application in detecting infectious and metabolic disorders.
- By Application (Infectious Diseases, Oncology, Endocrinology, and Cardiology): The infectious diseases segment is projected to reach USD 7.55 billion by 2032, fueled by the rising prevalence of viral and bacterial infections.
- By End-User (Hospitals & Clinics, Diagnostic Laboratories, Research & Academic Institutes, and Pharmaceutical & Biotechnology Companies): The hospitals & clinics segment is expected to register a share of 39.62% by 2032, propelled by their major role as primary diagnostic centers.
Chemiluminescence Immunoassay Market Regional Analysis
Based on region, the market has been classified into North America, Europe, Asia Pacific, Middle East & Africa, and South America.
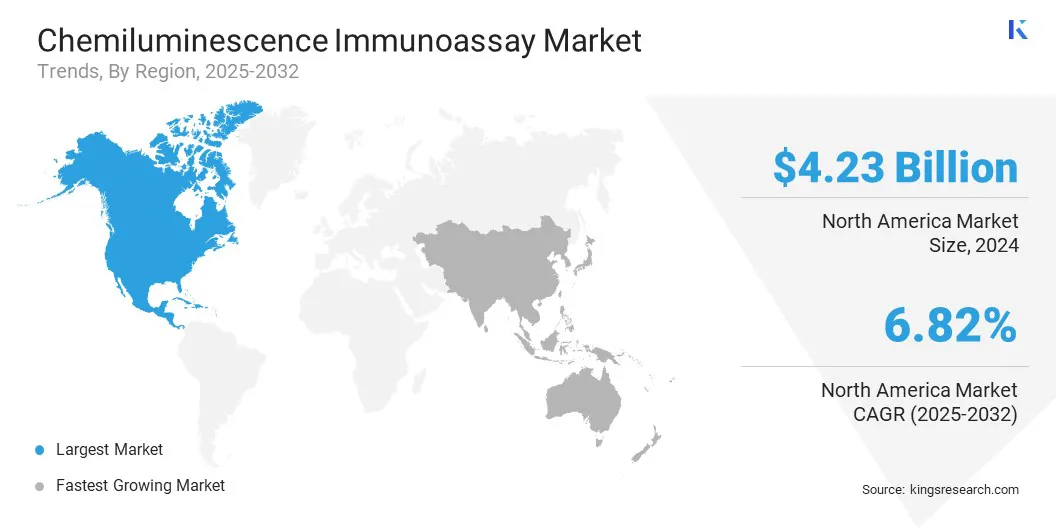
North America chemiluminescence immunoassay market share stood at 6.82% in 2024, valued at USD 4.23 billion. This dominance is primarily reinforced by the high prevalence of chronic and infectious diseases, advanced healthcare infrastructure, and strong adoption of automated diagnostic platforms.
- According to the Centers for Disease Control and Prevention (CDC), six in ten adults in the U.S. have a chronic disease, underscoring the substantial demand for reliable diagnostic testing.
Favorable reimbursement frameworks and advancements in healthcare innovation are further boosting adoption. Additionally, government initiatives such as the U.S. Department of Health and Human Services’ (HHS) programs to strengthen laboratory capacity are fostering regional market expansion.
The Asia-Pacific chemiluminescence immunoassay industry is poised to grow at a CAGR of 7.44% over the forecast period. This growth is supported by rising healthcare expenditure, expanding diagnostic infrastructure, and increasing awareness of early disease detection. Countries in the region are witnessing growing demand for advanced immunoassay technologies due to the high incidence of infectious diseases and the rising burden of lifestyle-related disorders.
- According to the International Diabetes Federation (IDF), in 2024, approximately 215.4 million adults aged 20–79 in the Western Pacific Region were living with diabetes, highlighting the urgent need for accurate and accessible diagnostic testing.
The growing emphasis on efficiency, reliability, and scalability in clinical laboratories are further accelerating adoption of CLIA systems.
Regulatory Frameworks
- In North America, the U.S. Food and Drug Administration (FDA) regulates diagnostic assays, including CLIA systems, through a stringent regulatory approval process for instruments and reagents. The Centers for Medicare & Medicaid Services (CMS) also enforces the Clinical Laboratory Improvement Amendments (CLIA) regulations to ensure laboratory testing quality.
- In Europe, the European Medicines Agency (EMA) and in Vitro Diagnostic Regulation (IVDR) govern the approval, quality, and performance of immunoassay systems across member states.
- In APAC, regulatory frameworks vary by country, with China’s National Medical Products Administration (NMPA) strengthening evaluation processes for diagnostic devices and Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) enforcing strict in vitro diagnostics guidelines.
- Globally, the World Health Organization (WHO) promotes harmonization of diagnostic standards and provides guidance on laboratory practices in resource-limited settings.
Competitive Landscape
Leading players operating in the global chemiluminescence immunoassay industry are emphasizing the development of fully automated, high-throughput analyzers that reduce turnaround time and manage increasing test volumes. Strategic mergers, acquisitions, and collaborations are being implemented to expand product portfolios and strengthen geographic presence.
Additionally, manufacturers are incorporating advanced analytics and digital technologies within CLIA platforms to improve diagnostic precision and workflow efficiency. Investment in expanding reagent menus across multiple therapeutic areas, including oncology, endocrinology, and infectious diseases, remains a key strategy to reinforce market position.
- In May 2025, Revvity launched the IDS i20 analytical random access platform from EUROIMMUN, a CE-marked and FDA-listed system that automated chemiluminescence immunoassays (ChLIA). The platform integrates multiple specialty tests on a single instrument, offering higher reagent capacity and throughput than existing devices.
Key Companies in Chemiluminescence Immunoassay Market:
- Hoffmann-La Roche Ltd
- Siemens Medical Solutions USA, Inc.
- DH Life Sciences, LLC.
- Autobio
- DiaSorin S.p.A.
- Mindray Medical India Pvt. Ltd.
- Beckman Coulter, Inc.
- Ozelle
- Nanjing Poclight Biotechnology Co., Ltd.
- Biovantion Inc.
- Zecen Biotech CO., LTD.
- Sysmex Corporation
- Shenzhen New Industries Biomedical Engineering Co., Ltd.
-
Diatron.
- Getein Biotech, Inc.
Recent Developments
- In January 2025, Anbio Biotechnology launched the Dry Chemiluminescence Immunoassay (CLIA) Solution ADL-1000, offering rapid, reliable, and cost-effective diagnostics for diverse clinical applications.


