Market Definition
The market includes a broad range of solutions and tools used to separate cells from tissues or culture surfaces. It supports key applications such as tissue dissociation and cell detachment. The market covers enzymatic and non-enzymatic reagents, along with instruments and accessories that are crucial in the dissociation process.
These offerings are used across pharmaceutical and biotechnology companies, along with research and academic institutes. The report provides a comprehensive analysis of key drivers, emerging trends, and the competitive landscape expected to influence the market over the forecast period.
Cell Dissociation Market Overview
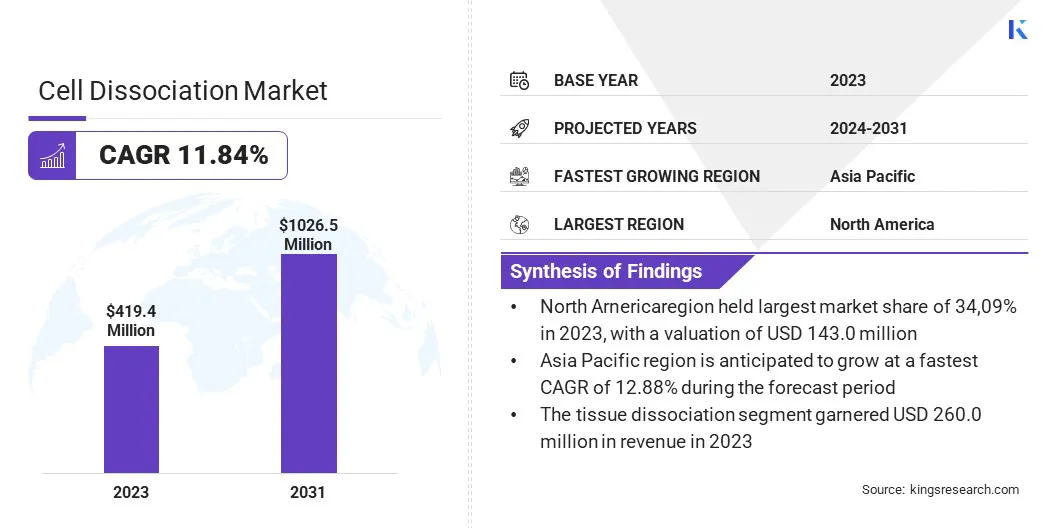
The global cell dissociation market size was valued at USD 419.4 million in 2023 and is projected to grow from USD 468.9 million in 2024 to USD 1026.5 million by 2031, exhibiting a CAGR of 11.84% during the forecast period.
Market growth is driven by the increasing demand for cell-based research, personalized medicine, and regenerative therapies. The rising adoption of cell culture techniques across pharmaceutical, biotechnology, and academic research institutes is further highlighting the need for effective and efficient cell dissociation solutions.
Major companies operating in the cell dissociation industry are Avantor, Inc., F. Hoffmann-La Roche Ltd, Lonza Group Ltd., Abcam Limited, Santa Cruz Biotechnology, Inc., Agilent Technologies, Inc., Thermo Fisher Scientific Inc., Becton, Dickinson and Company, Promega Corporation, Cytiva, Merck KGaA, STEMCELL Technologies, Corning Incorporated, Sartorius AG, and Worthington Biochemical Corporation.
Additionally, advancements in non-enzymatic dissociation technologies are fueling market growth by providing methods that maintain cell integrity, making them more suitable for sensitive applications. The increasing focus on biologics, including gene therapies and immunotherapies, is further contributing to this expansion.
- In April 2025, Thermo Fisher Scientific Inc. announced its Advanced Therapies Collaboration Center in California. The 6,000-square-foot facility aims to accelerate the development and commercialization of cell therapies, providing support to biopharma, biotech, and translational customers focused on cell-based immunotherapies.
Key Highlights:
- The cell dissociation market size was recorded at USD 419.4 million in 2023.
- The market is projected to grow at a CAGR of 11.84% from 2024 to 2031.
- North America held a market share of 34.09% in 2023, with a valuation of USD 143.0 million.
- The enzymatic dissociation segment garnered USD 161.3 million in revenue in 2023.
- The tissue dissociation segment is expected to reach USD 624.5 million by 2031.
- The pharmaceutical & biotechnology companies segment is projected to generate a value of USD 560.6 million by 2031.
- Asia Pacific is anticipated to grow at a CAGR of 12.88% during the forecast period.
Market Driver
Increasing Investment in Biologics and Drug Discovery
The market is witnessing significant growth due to increasing investment in biologics and drug discovery. As pharmaceutical and biotechnology companies expand their pipelines for monoclonal antibodies, cell therapies, and vaccines, there is a rising need for efficient cell isolation and processing methods.
Cell dissociation is a critical step in upstream workflows for biologics production, where maintaining cell viability and functionality impacts research outcomes and therapeutic yields. This has led to the greater adoption of high-quality dissociation reagents and instruments that support consistent cell recovery, reduce batch variability, and align with GMP requirements.
- In April 2025, Novartis announced plans to invest USD 23 billion over the next five years to expand its U.S.-based manufacturing and R&D operations. This will fund the development of 10 facilities, including 7 new ones, enabling Novartis to produce all its key medicines in the U.S. Key initiatives include the establishment of a biomedical research innovation hub in San Diego and new manufacturing facilities for biologics, chemical drug substances, and radioligand therapy.
Market Challenge
Inconsistent Performance of Dissociation Reagents
A major challenge hampering the expansion of the cell dissociation market is the inconsistent performance of dissociation reagents across different cell types. Enzymatic dissociation methods can cause variability in cell yield, viability, and function, particularly when working with sensitive or complex cell populations such as stem cells.
This inconsistency can affect the reproducibility and reliability of research findings, posing risks in both academic and pharmaceutical research. This challenge can be addressed through the development of more refined, cell-specific dissociation reagents and automated systems that can standardize the dissociation process.
Market Trend
Rising Shift Toward Non-Enzymatic Dissociation Solutions
The market is witnessing a growing preference for non-enzymatic dissociation solutions. This trend is supported by the need to preserve cell surface integrity and maintain consistency in sensitive research workflows. Non-enzymatic reagents help reduce cellular stress and improve viability, making them suitable for stem cell research, immunology, and therapy development.
This is leading to the increased adoption of these solutions by pharmaceutical, biotechnology, and academic sectors that prioritize reproducibility and high-quality results. The demand for xeno-free, defined formulations is rising, aligning with industry trends toward safer, more reliable dissociation methods in preclinical and clinical settings.
- In February 2023, TheWell Bioscience Inc. launched VitroGel Organoid Recovery Solution. This non-enzymatic recovery solution is designed for 3D cell cultures and organoids. It enables rapid 2-minute dissociation of animal-based ECM, providing a high recovery rate and cell viability for intact cells. The solution supports cell recovery from both animal-based ECM and VitroGel hydrogels, with complete dissociation under 15 minutes.
Cell Dissociation Market Report Snapshot
|
Segmentation
|
Details
|
|
By Product
|
Enzymatic Dissociation (Trypsin, Collagenase, Elastase, Papain, Hyaluronidase, DNase, Others), Non-enzymatic Dissociation, Instruments & Accessories
|
|
By Application
|
Tissue Dissociation, Cell Detachment
|
|
By End User
|
Pharmaceutical & Biotechnology Companies, Research & Academic Institutes
|
|
By Region
|
North America: U.S., Canada, Mexico
|
|
Europe: France, UK, Spain, Germany, Italy, Russia, Rest of Europe
|
|
Asia-Pacific: China, Japan, India, Australia, ASEAN, South Korea, Rest of Asia-Pacific
|
|
Middle East & Africa: Turkey, U.A.E., Saudi Arabia, South Africa, Rest of Middle East & Africa
|
|
South America: Brazil, Argentina, Rest of South America
|
Market Segmentation
- By Product (Enzymatic Dissociation (Trypsin, Collagenase, Elastase, Papain, Hyaluronidase, DNase, Others) , Non-enzymatic Dissociation, Instruments & Accessories): The enzymatic dissociation segment earned USD 161.3 million in 2023, mainly due to its efficiency in isolating viable cells from complex tissue structures.
- By Application (Tissue Dissociation and Cell Detachment): The tissue dissociation held a share of 62.01% in 2023, owing to the rising demand for primary cell isolation in disease modeling and drug screening.
- By End User (Pharmaceutical & Biotechnology Companies, and Research & Academic Institutes): The pharmaceutical & biotechnology companies segment is projected to reach USD 560.6 million by 2031, on account of the increased investments in cell-based research and biologics development.
Cell Dissociation Market Regional Analysis
Based on region, the global market has been classified into North America, Europe, Asia Pacific, Middle East & Africa, and South America.
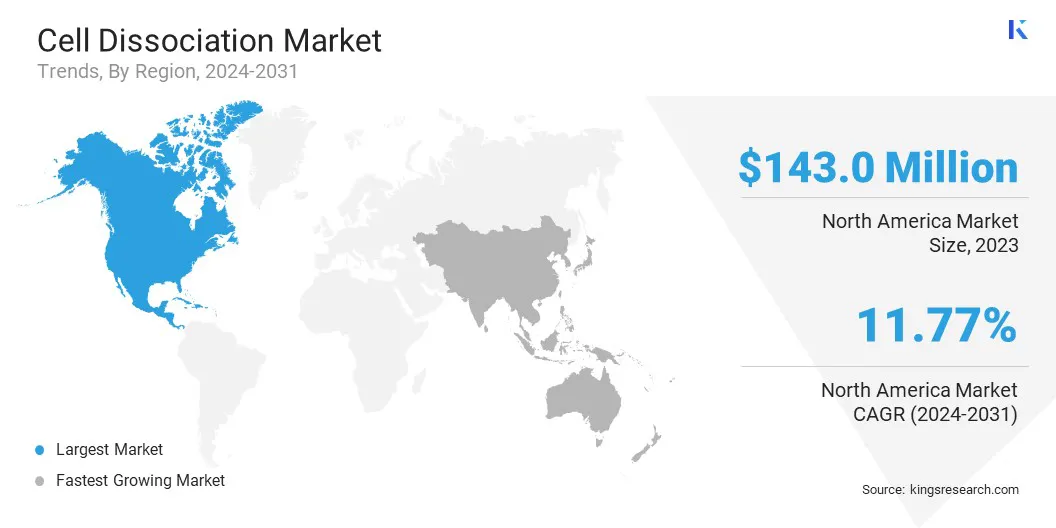
The North America cell dissociation market share stood at around 34.09% in 2023, valued at USD 143.0 million. This dominance is reinforced by well-established pharmaceutical and biotechnology sectors, supported by strong funding and advanced research infrastructure.
Leading companies and academic institutions in the region continue to adopt advanced dissociation tools across drug discovery, stem cell research, and regenerative medicine. Regulatory frameworks that promote innovation in biologics and cell therapy have boosted regional market expansion.
The Asia Pacific cell dissociation industry is poised to grow at a significant CAGR of 12.88% over the forecast period. This growth is supported by rising investments in biopharmaceutical manufacturing, expanding academic research, and government-backed initiatives in life sciences.
Countries such as China, India, and Japan are enhancing local capabilities in cell-based research and development and clinical trials. The growing focus on regenerative medicine, personalized therapy, and vaccine development is accelerating the adoption of dissociation solutions. Increasing collaborations with global firms and the expansion of local biotech companies are further bolstering regional market growth.
- In February 2024, Miltenyi Biotec launched its first office in India and unveiled plans to set up the Miltenyi Innovation and Technology Center as a Cell and Gene Therapy (CGT) Centre of Excellence (COE) in Hyderabad. This COE will offer specialized training in cell and gene therapy, covering the entire process from proof-of-concept to clinical development and commercialization, while providing researchers and clinicians easier access to expertise and manufacturing solutions.
Regulatory Frameworks
- In the U.S., the regulatory framework for cell dissociation is primarily governed by the Food and Drug Administration (FDA), which oversees the approval of biologics and medical devices used in cell therapies. Cell dissociation reagents and related products fall under the FDA’s oversight, particularly if they are intended for clinical or research use.
- In Europe, the European Medicines Agency (EMA) regulates cell dissociation products, particulaly those involved in cell-based medicinal products. The European Commission’s Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR) govern products used in diagnostic and therapeutic applications related to cell dissociation.
- In India, the Central Drugs Standard Control Organization (CDSCO) regulates the manufacturing and distribution of cell dissociation products, particularly those used in clinical settings. Companies must comply with the Good Manufacturing Practice (GMP) and safety standards established by the CDSCO.
Competitive Landscape
Companies operating in the cell dissociation market are focusing on product innovation and technological advancements to improve the efficacy and efficiency of dissociation reagents and instruments. Many are investing in R&D to develop new and more efficient solutions for cell separation, catering to diverse research and clinical applications, particularly in cell-based therapies and regenerative medicine.
Strategic partnerships and collaborations with academic institutions, research organizations, and contract research organizations (CROs) are common, helping expand product offerings and enhance access to emerging markets.
Through these collaborations, companies can leverage external expertise to accelerate the development of novel technologies and improve product performance.
Additionally, companies are implementing acquisitions and mergers as a strategy to broaden their product portfolio and increase their market footprint. Acquiring smaller, innovative companies with niche technologies allows larger firms to quickly enter new market segments and enhance their technological capabilities.
- In March 2023, Agilent Technologies Inc. acquired e-MSion, developer of the ExD cell electron capture dissociation technology. The acquisition will enable Agilent to integrate the ExD cell into its portfolio, providing biopharma researchers with enhanced capabilities to accelerate drug development.
List of Key Companies in Cell Dissociation Market:
- Avantor, Inc.
- F. Hoffmann-La Roche Ltd
- Lonza Group Ltd.
- Abcam Limited
- Santa Cruz Biotechnology, Inc.
- Agilent Technologies, Inc.
- Thermo Fisher Scientific Inc.
- Becton, Dickinson and Company
- Promega Corporation
- Cytiva
- Merck KGaA
- STEMCELL Technologies
- Corning Incorporated
- Sartorius AG
- Worthington Biochemical Corporation
Recent Developments (Product Launch)
- In September 2024, Singleron launched the sCelLiVE Tissue Dissociation Kit (Skin) designed for mouse and human skin samples. The kit reduces processing time from 3 hours to 60–120 minutes, enabling downstream applications such as single-cell sequencing.


