Market Definition
Cardiac marker testing involves measuring specific proteins released into the bloodstream following heart injury. Key biomakers include troponin, creatine kinase-MB, and B-type natriuretic peptide, which serve as early indicators of cardiac events such as myocardial infarction or heart failure.
The market includes hospital laboratories, diagnostic centers, and point-of-care settings supporting diagnosis, timely intervention, patient monitoring, and improved clinical decision-making in critical cardiac care.
Cardiac Marker Testing Market Overview
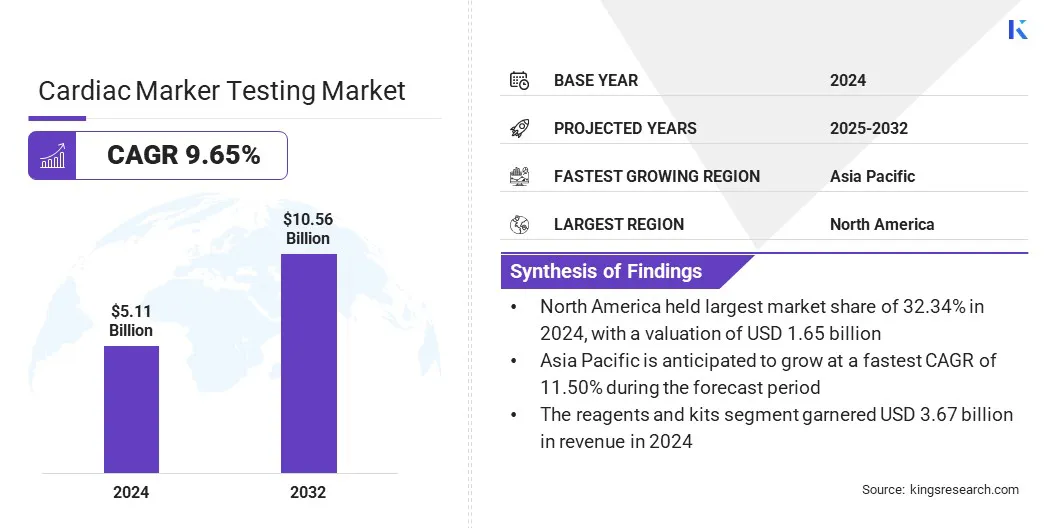
According to Kings Research, the global cardiac marker testing market size was valued at USD 5.11 billion in 2024 and is projected to grow from USD 5.54 billion in 2025 to USD 10.56 billion by 2032, exhibiting a CAGR of 9.65% during the forecast period.
The growth of the market is driven by the shift toward point-of-care testing, which enables faster diagnosis and treatment in emergency and outpatient settings. This growth is further supported by the integration of AI and data analytics, which enhances test interpretation and supports improved clinical decision-making for cardiovascular care.
Key Highlights
- The cardiac marker testing industry size was USD 5.11 billion in 2024.
- The market is projected to grow at a CAGR of 9.65% from 2025 to 2032.
- North America held a share of 38.42% in 2024, valued at USD 1.96 billion.
- The troonins I and T segment garnered USD 1.65 billion in revenue in 2024.
- The reagents & kits segment is expected to reach USD 7.35 billion by 2032.
- The myocardial infarction segment secured the largest revenue share of 35.55% in 2024.
- The point-of-care (POC) facilities segment is set to grow at a robust CAGR of 10.74% through the forecast period.
- Asia Pacific is anticipated to grow at a CAGR of 11.80% over the forecast period.
Major companies operating in the cardiac marker testing market are Abbott, F. Hoffmann-La Roche Ltd, Siemens, Danaher Corporation, BIOMÉRIEUX, BD, Beckman Coulter, Inc., Bio-Rad Laboratories, Inc, Thermo Fisher Scientific Inc., QuidelOrtho Corporation, Agilent Technologies, Inc., Fujifilm Group, DiaSorin S.p.A., PerkinElmer, and Randox Laboratories Ltd.
The market is witnessing significant growth, mainly due to the increasing prevalence of heart attacks, heart failure, and other cardiovascular disorders requiring timely diagnosis and monitoring.
- In October 2024, the U.S. Centers for Disease Control and Prevention reported that in 2023, cardiovascular disease caused approximately 919,032 deaths in the U.S., which is one in every three deaths, and that around 805,000 Americans suffered a heart attack, including 605,000 first-time attacks and 200,000 recurrent events.
Cardiac marker testing is essential for detecting biochemical changes associated with myocardial injury and disease progression. Hospitals and diagnostic centers are increasingly relying on these tests to provide faster and more accurate clinical decisions. Growing awareness of preventive healthcare is prompting individuals at high risk to undergo regular cardiac marker testing. Rising healthcare expenditure and expanded access to diagnostic services are further supporting adoption.
Market Driver
Rising Demand for Point-of-Care Testing
The growth of the cardiac marker testing market is driven by the rising demand for rapid diagnostic solutions in emergency departments and critical care units where timely decisions are vital. Point-of-care devices enable faster detection of conditions such as myocardial infarction, reducing the need for central lab processing.
Portable systems improve accessibility in remote or resource-limited healthcare settings, supporting prompt patient management. Physicians are increasingly adopting these tools to streamline workflows and enhance treatment efficiency. The growing preference for decentralized testing is creating strong opportunities for device manufacturers.
- In June 2024, Katherine Hospital in the Northern Territory, Australia, became the first hospital in the country to implement the Siemens Healthineers Atellica VTLi point-of-care cardiac marker system. This device delivers initial heart attack diagnoses from a simple finger-prick blood test, providing results in eight minutes, significantly faster than the previous six-hour wait for laboratory results.
Market Challenge
High Cost of Advanced Assays
A key challenge impeding the progress of the cardiac marker testing market is the high expense associated with advanced assays, particularly high-sensitivity troponin and related tests. These tests require sophisticated instruments and consumables, which increase the overall cost of diagnosis. Furthermore, limited reimbursement policies and constrained healthcare budgets in low-resource settings restrict accessibility to such advanced diagnostic solutions.
To address this challenge, market players are developing cost-efficient assay platforms, expanding point-of-care solutions, and collaborating with healthcare systems to improve affordability. These strategies enable broader adoption of cardiac marker testing across both advanced and resource-constrained healthcare environments.
Market Trend
Integration of AI & Data Analytics
The cardiac marker testing market is increasingly incorporating artificial intelligence and advanced data analytics to enhance diagnostic accuracy. AI-driven algorithms assist in interpreting complex biomarker patterns, enabling earlier detection of cardiac events and better stratification of patient risk.
Data analytics platforms integrate test results with electronic health records and clinical parameters, supporting evidence-based decision-making for physicians. This approach is improving efficiency in emergency care, personalized treatment planning, and long-term disease monitoring. By reducing diagnostic uncertainty and enabling predictive insights, AI and analytics are strengthening the role of cardiac marker testing in modern healthcare.
- In December 2024, University of California, Los Angeles (UCLA) researchers developed a deep learning–powered chemiluminescence vertical flow assay (CL-VFA), enabling high-sensitivity cardiac troponin I testing at the point of care. This assay integrates a paper-based chemiluminescent sensor, a portable reader, and an AI-driven analysis algorithm.
Cardiac Marker Testing Market Report Snapshot
|
Segmentation
|
Details
|
|
By Biomarker
|
Troonins I and T, Creatine Kinase-MB, Brain Natriuretic Peptide, Myoglobin, High-sensitivity C-reactive Protein, Others
|
|
By Product
|
Reagents & Kits, Instruments
|
|
By Indication
|
Myocardial Infarction, Congestive Heart Failure (CHF), Atherosclerosis, Acute Coronary Syndrome, Others
|
|
By End User
|
Laboratory Testing Facilities, Point-of-Care (POC) Facilities, Academic Institutes
|
|
By Region
|
North America: U.S., Canada, Mexico
|
|
Europe: France, UK, Spain, Germany, Italy, Russia, Rest of Europe
|
|
Asia-Pacific: China, Japan, India, Australia, ASEAN, South Korea, Rest of Asia-Pacific
|
|
Middle East & Africa: Turkey, U.A.E., Saudi Arabia, South Africa, Rest of Middle East & Africa
|
|
South America: Brazil, Argentina, Rest of South America
|
Market Segmentation
- By Biomarker (Troonins I and T, Creatine Kinase-MB, Brain Natriuretic Peptide, Myoglobin, High-sensitivity C-reactive Protein, and Others): The troonins I and T segment earned USD 1.65 billion in 2024, mainly due to their high sensitivity and specificity in diagnosing myocardial infarction and their central role in clinical guidelines for acute cardiac care.
- By Product (Reagents & Kits, Instruments): The reagents & kits segment held a share of 71.77% in 2024, owing to their consistent demand across diagnostic procedure, ensuring steady revenue and widespread use in both central laboratories and point-of-care settings.
- By Indication (Myocardial Infarction, Congestive Heart Failure (CHF), Atherosclerosis, Acute Coronary Syndrome, and Others): The myocardial infarction segment is projected to reach USD 3.71 billion by 2032, supported by the reliance on rapid and accurate biomarker assays for early diagnosis and timely intervention in acute cardiac events.
- By End User (Laboratory Testing Facilities, Point-of-Care (POC) Facilities, and Academic Institutes): The point-of-care (POC) facilities segment is estimated to grow at a CAGR of 10.74% through the forecast period, attributed to the rising demand for rapid diagnostic results that enable timely treatment decisions in emergency and outpatient settings.
Cardiac Marker Testing Market Regional Analysis
Based on region, the market has been classified into North America, Europe, Asia Pacific, Middle East & Africa, and South America.
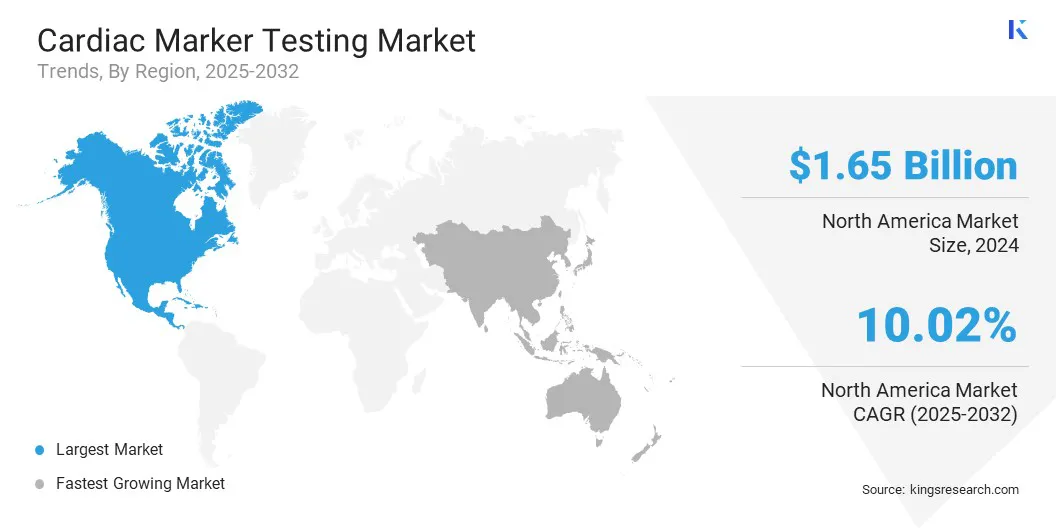
North America cardiac marker testing market share stood at 38.42% in 2024, valued at USD 1.96 billion. This dominance is reinforced by the rising adoption of point-of-care cardiac marker devices in ambulances, urgent care centers, and outpatient facilities.
Clinicians in North America prefer portable testing solutions to accelerate patient triage outside central labs. These devices reduce the time between symptom onset and diagnosis, particularly in emergency and rural settings. The demand for faster interventions is prompting greater reliance on cardiac marker testing at the point of care.
- In March 2024, Polymedco received 510(k) clearance from the U.S. Food and Drug Administration for its rapid high-sensitivity troponin I assay (hs-cTnI-II) on the Pathfast Biomarker Analyzer, enabling faster point-of-care detection of myocardial infarction. This platform delivers results in 17 minutes, significantly faster than traditional central lab processing.
The Europe cardiac marker testing industry is set to grow at a significant CAGR of 11.80% over the forecast period. This growth is stimulated by the rising prevalence of heart disease and related conditions, fueled by lifestyle changes and increasing rates of diabetes and hypertension. Hospitals are witnessing a growing number of emergency visits for chest pain and suspected heart attacks, which is boosting demand for accurate cardiac marker testing.
Additionally, governments and private investments are leading to the development of advanced healthcare facilities in urban and semi-urban areas, equipped with modern diagnostic technologies, including cardiac marker analyzers. The expanded infrastructure is further supporting the adoption of these tests.
- In 2024 , Roche Diagnostics expanded its presence in the Asia-Pacific region by establishing a cardiac point-of-care (POC) production and training center in Singapore. This facility supports regional distribution of portable cardiac testing devices and enhances local clinician training.
Regulatory Frameworks
- In the U.S., cardiac marker tests are regulated as in-vitro diagnostic devices by the Food and Drug Administration (FDA). Most assays undergo either the 510(k) premarket clearance process or Premarket Approval (PMA), depending on risk. The Centers for Medicare and Medicaid Services (CMS) oversees laboratory certification under the Clinical Laboratory Improvement Amendments (CLIA).
- In the EU, cardiac marker assays fall under the In Vitro Diagnostic Regulation (IVDR, EU 2017/746), which came into effect in May 2022. The regulation applies a risk-based classification system (Classes A–D), with cardiac markers usually requiring assessment by a Notified Body. Manufacturers must demonstrate clinical performance, maintain post-market surveillance, and register products in the European Database on Medical Devices (EUDAMED).
- In Japan, cardiac marker tests are regulated by the Ministry of Health, Labour and Welfare (MHLW) and reviewed by the Pharmaceuticals and Medical Devices Agency (PMDA). Manufacturers must submit evidence of safety, analytical performance, and clinical validity for approval. Depending on risk classification, cardiac marker assays may undergo full premarket review.
- In China, cardiac marker testing is monitored by the National Medical Products Administration (NMPA). In-vitro diagnostics are classified into three categories based on risk, with cardiac assays generally placed in Class II or III. These products must undergo technical evaluation and may require local clinical trials.
- In India, cardiac marker devices are governed by the Medical Devices Rules 2017 under the Central Drugs Standard Control Organisation (CDSCO). Approval requires evidence of analytical performance, stability, and clinical reliability. The Indian Council of Medical Research (ICMR) issues protocols for evaluating diagnostic kits and collaborates with CDSCO to designate performance evaluation laboratories.
Competitive Landscape
Major players in the cardiac marker testing industry are developing high-sensitivity assays, expanding point-of-care solutions, and advancing analyzer platforms to maintain competitiveness. Companies are investing in research and development to introduce faster and more accurate biomarker tests that address the need for early diagnosis.
Partnerships with hospitals and emergency care providers are strengthening product reach in decentralized and resource-limited settings. Additionally, diagnostic platforms are being upgraded for seamless integration across central labs, clinics, and mobile healthcare units. These strategies support the growing demand for rapid cardiac testing and help maintain a strong market position.
- In June 2024, Siemens Healthineers added the NT-proBNPII assay to its Atellica Solution platform for diagnosing heart failure. It provides a 10-minute time-to-first-result and supports usage on Atellica IM or CI analyzers. It can be deployed in ambulances or decentralized clinics to support swift triage where lab infrastructure is limited.
Key Companies in Cardiac Marker Testing Market:
- Abbott
- Hoffmann-La Roche Ltd
- Siemens
- Danaher Corporation
- BIOMÉRIEUX
- BD
- Beckman Coulter, Inc.
- Bio-Rad Laboratories, Inc
- Thermo Fisher Scientific Inc.
- QuidelOrtho Corporation
- Agilent Technologies, Inc.
- Fujifilm Group
- DiaSorin S.p.A.
- PerkinElmer
- Randox Laboratories Ltd.
Recent Developments (Approval/Product Launch)
- In October 2024, Siemens Healthineers received FDA clearance for a new prognostic claim on its Atellica IM High-Sensitivity Troponin I test (TnIH). This high-sensitivity troponin test is the first in the U.S. that can predict the risk of cardiac events or mortality up to one year after presentation, aiding in long-term care planning.
- In October 2023, Mindray launched new high-sensitivity cardiac biomarker assays: hs-cTnI and NT-proBNP. These assays deliver high precision, with the NT-proBNP test showing resistance to interference from common cardiac medications, enhancing early detection and management of cardiovascular conditions.


