Market Definition
The market encompasses the ecosystem involved in monitoring, detecting, and managing antimicrobial resistance (AMR) across healthcare and public health settings. It includes solutions and services that support the identification and reporting of resistant pathogens.
The market covers diagnostic kits & systems used to analyze clinical samples, surveillance software that enables data collection & integration, and services that facilitate laboratory operations, reporting, and epidemiological analysis. The report offers a thorough assessment of the main factors driving the market, along with detailed regional analysis and the competitive landscape influencing market dynamics.
Antimicrobial Resistance Surveillance Market Overview
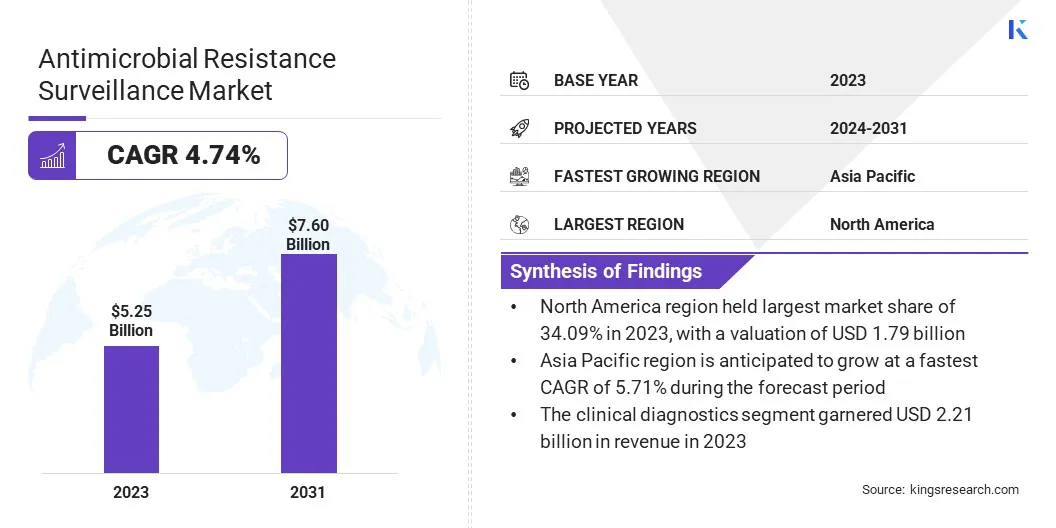
The global antimicrobial resistance surveillance market size was valued at USD 5.25 billion in 2023 and is projected to grow from USD 5.49 billion in 2024 to USD 7.60 billion by 2031, exhibiting a CAGR of 4.74% during the forecast period.
The market is driven by the prevalence of antimicrobial resistance, expansion of national and international AMR surveillance programs, growing public health awareness, and the adoption of advanced diagnostic technologies, including genome sequencing and rapid AST platforms. Increasing collaboration between global health organizations and private stakeholders is further supporting the market expansion.
Major companies operating in the antimicrobial resistance surveillance industry are Pfizer Inc., Abbott, OpGen, BIOMÉRIEUX, Hologic, Inc., Charles River Laboratories, F. Hoffmann-La Roche Ltd, Accelerate Diagnostics, Inc., Bio-Rad Laboratories, Inc., Thermo Fisher Scientific Inc., Oxford Nanopore Technologies plc., Illumina, Inc., Becton, Dickinson and Company, Liofilchem S.r.l, and QIAGEN.
Additionally, the integration of AI into diagnostic systems and antimicrobial resistance surveillance platforms is significantly enhancing the efficiency and accuracy of detecting resistant strains. AI algorithms can quickly analyze large datasets, identifying patterns and trends that may not be immediately apparent through traditional methods.
- In April 2023, the UK Health Security Agency (UKHSA) launched a nationwide surveillance study to assess levels of AMR in healthy individuals. The initiative aims to collect samples from up to 2,000 participants to better understand the prevalence and drivers of antibiotic-resistant bacteria in the general population, including CPE, ESBLs, MRSA, and Candida auris.
Key Highlights:
- The antimicrobial resistance surveillance market size was valued at USD 5.25 billion in 2023.
- The market is projected to grow at a CAGR of 4.74% from 2024 to 2031.
- North America held a market share of 34.09% in 2023, with a valuation of USD 1.79 billion.
- The diagnostic kits segment garnered USD 1.96 billion in revenue in 2023.
- The clinical diagnostics segment is expected to reach USD 3.17 billion by 2031.
- The hospitals & clinics segment is expected to reach USD 2.93 billion by 2031.
- The market in Asia Pacific is anticipated to grow at a CAGR of 5.71% during the forecast period.
Market Driver
Rising Prevalence of Antimicrobial Resistance
The global market is driven by the increasing incidence of AMR. The rise in resistant infections has created a pressing need for effective surveillance systems. AMR is contributing to longer hospital stays, higher treatment costs, and greater mortality rates.
As a result, healthcare organizations and governments are focusing on improving surveillance to track resistance patterns and inform public health strategies. This shift drives the demand for advanced diagnostic tools and surveillance solutions, fueling the market.
- In February 2025, the Centers for Disease Control and Prevention (CDC) reported that more than 2.8 million antimicrobial-resistant infections occur annually in the U.S., highlighting AMR as an urgent global public health threat.
Market Challenge
Lack of Standardized Data Collection
A major challenge in the antimicrobial resistance surveillance market is the lack of standardized data collection and reporting. Variability in data quality, reporting protocols, and surveillance methods can hinder the effectiveness of AMR monitoring systems.
This inconsistency makes it difficult to compile comprehensive global datasets, which are crucial for tracking resistance patterns and implementing effective public health strategies. A potential solution to this challenge is the establishment of global standards for AMR surveillance, supported by international collaborations and technological platforms.
Standardized guidelines and integrated systems can ensure more accurate and consistent data reporting, improving the overall effectiveness of AMR surveillance programs.
Market Trend
Integration of Artificial Intelligence
One of the trends in the global market is the increasing integration of Artificial Intelligence (AI). AI is optimizing surveillance systems by enabling faster, more accurate identification of resistance patterns.
AI enhances the processing of large datasets from diagnostic tools and healthcare facilities through advanced data analytics, allowing for real-time monitoring and predictive insights. This integration improves operational efficiency, accelerates decision-making, and strengthens the overall effectiveness of AMR surveillance programs, providing a competitive edge in managing AMR.
- In May 2024, research by the Chinese Academy of Sciences and Newcastle University revealed critical gaps in global AMR research. The research, supported by AI, identified knowledge, methodological, and communication gaps, emphasizing the need for greater coordination across sectors and regions to effectively combat AMR.
Antimicrobial Resistance Surveillance Market Report Snapshot
|
Segmentation
|
Details
|
|
By Component
|
Diagnostic Kits, Diagnostic Systems, Surveillance Software, Services
|
|
By Application
|
Clinical Diagnostics, Public Health Surveillance, Others
|
|
By End User
|
Hospitals & Clinics, Research & Academic Institutes, Others
|
|
By Region
|
North America: U.S., Canada, Mexico
|
|
Europe: France, UK, Spain, Germany, Italy, Russia, Rest of Europe
|
|
Asia-Pacific: China, Japan, India, Australia, ASEAN, South Korea, Rest of Asia-Pacific
|
|
Middle East & Africa: Turkey, U.A.E., Saudi Arabia, South Africa, Rest of Middle East & Africa
|
|
South America: Brazil, Argentina, Rest of South America
|
Market Segmentation:
- By Component (Diagnostic Kits, Diagnostic Systems, Surveillance Software, Services): The diagnostic kits segment earned USD 1.96 billion in 2023, due to the rising demand for rapid and accurate detection of resistant pathogens in clinical settings.
- By Application (Clinical Diagnostics, Public Health Surveillance, Others): The clinical diagnostics segment held 42.17% share of the market in 2023, due to the growing prevalence of AMR and the need for early intervention.
- By End User (Hospitals & Clinics, Research & Academic Institutes, Others): The hospitals & clinics segment is projected to reach USD 2.93 billion by 2031, owing to the increased adoption of AMR surveillance tools to improve patient outcomes and infection control practices.
Antimicrobial Resistance Surveillance Market Regional Analysis
Based on region, the global market has been classified into North America, Europe, Asia Pacific, Middle East & Africa, and South America.
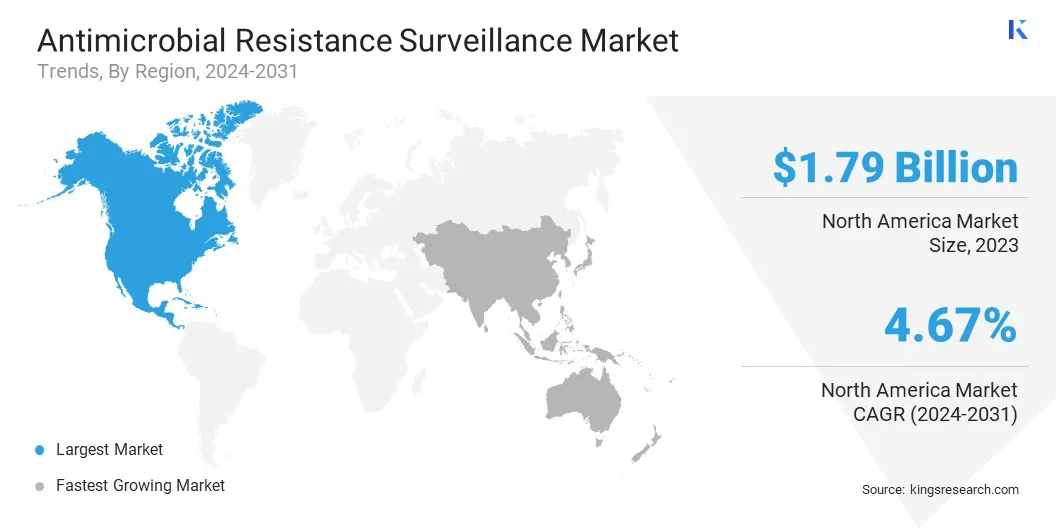
North America accounted for 34.09% share of the antimicrobial resistance surveillance market in 2023, with a valuation of USD 1.79 billion. This market dominance is attributed to the presence of well-established healthcare infrastructure, high adoption of advanced diagnostic systems, and strong government initiatives supporting AMR monitoring.
The region benefits from significant investments in research and public health programs, along with strict regulatory frameworks that promote surveillance compliance. Increased collaboration between public health agencies and private sector players has accelerated the development and deployment of surveillance technologies.
The region’s strong data integration capabilities and advanced health IT systems have enhanced the efficiency of AMR data collection and reporting.
The antimicrobial resistance surveillance industry in Asia Pacific is poised to grow at a significant CAGR of 5.71% over the forecast period, supported by increasing awareness of antimicrobial resistance and rising healthcare expenditure. The growing burden of infectious diseases and the emergence of drug-resistant pathogens have pushed countries in the region to adopt surveillance programs.
Governments are launching national action plans and collaborating with global health agencies to strengthen AMR tracking. Expanding diagnostic infrastructure, rising investment in laboratory capabilities, and growing support for digital health initiatives have contributed to the market’s expansion in this region.
- In March 2025, the Ministry of Health and Family Welfare, Government of India, strengthened efforts to combat AMR through multiple initiatives. The National Antimicrobial Resistance Surveillance Network (NARS-Net) was used to conduct AMR surveillance of 9 priority pathogens, analyzing clinical samples for defined drug-bug combinations.
Regulatory Frameworks
- In the U.S., the regulatory framework for AMR surveillance is primarily governed by the Centers for Disease Control and Prevention (CDC) through initiatives like the National Antimicrobial Resistance Monitoring System (NARMS). NARMS monitors trends in AMR among pathogens that affect humans, animals, and the environment.
- In India, the Indian Council of Medical Research (ICMR) leads AMR surveillance through the Integrated Disease Surveillance Program (IDSP). The ICMR, in collaboration with the World Health Organization (WHO), has established a national AMR surveillance network that collects data on resistance patterns from hospitals and healthcare centers across the country.
Competitive Landscape
Antimicrobial resistance surveillance market players are focusing on strategic partnerships and collaborations with public health organizations and research institutes to enhance their surveillance capabilities and broaden their data collection networks. They invest in research and development to innovate and improve diagnostic technologies, including the development of more accurate and rapid diagnostic kits & systems for monitoring AMR.
Another key strategy involves expanding geographic reach by entering emerging markets, where the need for AMR surveillance is increasing on account of rising health concerns. Players also engage in acquisitions to integrate complementary technologies and expand their product portfolios.
Companies focus on strengthening their digital infrastructure, integrating advanced data analytics and AI to improve the efficiency and accuracy of AMR surveillance programs.
- In November 2024, Cepheid and the Fleming Initiative announced a strategic partnership to address the global challenge of AMR. The collaboration, aligned with the UN General Assembly's high-level focus on AMR, aims to advance the use of in-vitro diagnostics to support antimicrobial stewardship and responsible antibiotic use.
List of Key Companies in Antimicrobial Resistance Surveillance Market:
- Pfizer Inc.
- Abbott
- OpGen
- BIOMÉRIEUX
- Hologic, Inc.
- Charles River Laboratories
- F. Hoffmann-La Roche Ltd
- Accelerate Diagnostics, Inc.
- Bio-Rad Laboratories, Inc.
- Thermo Fisher Scientific Inc.
- Oxford Nanopore Technologies plc.
- Illumina, Inc.
- Becton, Dickinson and Company
- Liofilchem S.r.l
- QIAGEN
Recent Developments (Funding)
- In April 2024, Selux Diagnostics, Inc. announced an additional USD 48 million in funding to support the U.S. commercialization of its Selux Next Generation Phenotyping (NGP) System. The platform delivers same-day Antibiotic Susceptibility Testing (AST) results, significantly reducing reliance on broad-spectrum antibiotics.


