Market Definition
Cartilage repair is the medical process of restoring damaged or degenerated joint cartilage to improve function, reduce pain, and prevent further joint deterioration. The market encompasses products, technologies, and services for diagnosis, treatment, and regeneration, including surgical techniques, biologics, tissue engineering, scaffolds, and post-operative rehabilitation solutions.
Global Cartilage Repair Market Overview
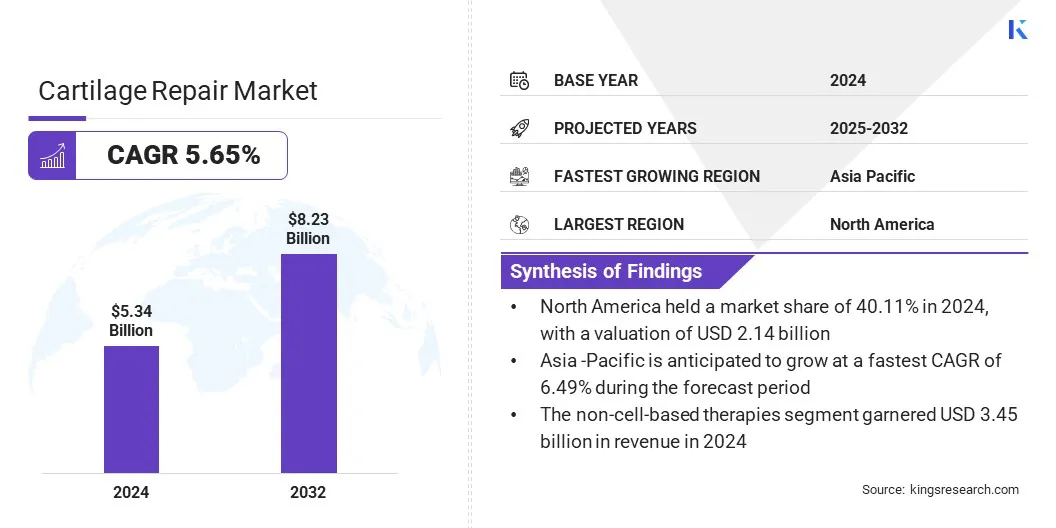
The global cartilage repair market size was valued at USD 5.34 billion in 2024 and is projected to grow from USD 5.60 billion in 2025 to USD 8.23 billion by 2032, exhibiting a CAGR of 5.65% during the forecast period.
The increasing number of patients suffering from osteoarthritis and sports-related knee, hip, and ankle injuries is creating a strong demand for effective cartilage repair therapies. The expanding elderly population is seeking treatments that preserve joint function and delay replacement, boosting the adoption of regenerative cartilage repair procedures.
Key Highlights:
- The cartilage repair industry size was recorded at USD 5.34 billion in 2024.
- The market is projected to grow at a CAGR of 5.65% from 2024 to 2032.
- North America held a share of 40.11% in 2024, valued at USD 2.14 billion.
- The non-cell-based therapies segment garnered USD 3.45 billion in revenue in 2024.
- The palliative segment is expected to reach USD 4.38 billion by 2032.
- The hip segment is anticipated to witness the fastest CAGR of 6.69% during the forecast period.
- The hospitals & clinics segment accounted for a share of 55.43% in 2024.
- Asia Pacific is anticipated to grow at a CAGR of 6.49% over the forecast period.
Major companies operating in the cartilage repair market are Vericel Corporation, Zimmer Biomet Holdings, Inc, Smith+Nephew, Arthrex, Inc, Stryker Corporation, B. Braun SE, CONMED Corporation, Anika Therapeutics, Inc, MEDIPOST Co., Ltd, Geistlich Pharma AG, Orthocell Ltd, Allosource, Collagen Solutions Ltd, Sparta Biomedical Inc, and TissueForm, Inc.
Technological innovation is fueling market growth through advanced materials, cell-based therapies, and scaffold technologies that enhance cartilage regeneration and improve outcomes. These innovations support minimally invasive procedures, faster recovery, and more effective joint repair, increasing adoption among healthcare providers and contributing to market growth.
- In March 2025, Drexel University received an NSF research grant to advance RibbonGel, a novel material designed to improve cartilage repair. The technology aims to create a stable 3D environment for cartilage cells and enhance healing and joint integration.
Market Driver
Rising Incidence of Sports and Recreational Injuries
A key factor propelling the growth of the cartilage repair market is the rising incidence of sports and recreational injuries. Increasing participation in physical activities and competitive sports is causing more joint damage and cartilage-related issues.
This is prompting patients to seek effective treatments that restore joint function, relieve pain, and prevent long-term degeneration. Increasing awareness of joint health, coupled with advances in minimally invasive and regenerative procedures, is accelerating the adoption of cartilage repair solutions.
- The National Safety Council reported that the number of sports and recreational injuries increased by 17% in 2024, rising to 564,845 cases compared to 482,886 in 2023. This is highlights a rising demand for cartilage repair treatments, as more patients require interventions to restore joint function and prevent long-term damage.
Market Challenge
High Cost of Advanced Cartilage Repair Procedures
A major challenge limiting the progress of the cartilage repair market is the high cost of advanced procedures, including cell-based therapies, tissue-engineered scaffolds, and hydrogel implants. These treatments require specialized surgical facilities, skilled healthcare professionals, and complex laboratory processes, which substantially increase expenses compared to conventional orthopedic interventions.
Additionally, ongoing costs for post-operative care, quality assurance, and patient monitoring contribute to the financial burden and limit accessibility and adoption of advanced cartilage repair procedures among patients and healthcare providers.
To address this challenge, market players are developing streamlined, minimally invasive therapies that reduce surgical time, facility requirements, and treatment expenses. They are also investing in scalable manufacturing processes for cell-based therapies and tissue-engineered products to lower production costs.
Additionally, collaborations with healthcare providers and initiatives to secure insurance coverage or reimbursement support improve patient access, making advanced regenerative treatments more affordable and widely adoptable.
Market Trend
Increasing Adoption of Minimally Invasive Procedures
A key trend influencing the cartilage repair market is the increasing adoption of minimally invasive procedures. Healthcare providers are utilizing arthroscopic techniques, hydrogel-based implants, and one-step cartilage repair technologies to reduce surgical trauma, shorten hospital stays, and accelerate patient recovery. These approaches provide faster rehabilitation, lower complication risks, and improved clinical outcomes for patients undergoing cartilage repair procedures.
- In April 2025, Hy2Care received FDA investigational device exemption (IDE) approval to initiate its first U.S. clinical trial for the CartRevive hydrogel implant. The implant is designed to repair traumatic knee cartilage injuries by providing a minimally invasive solution that addresses a significant unmet clinical need.
Global Cartilage Repair Market Report Snapshot
|
Segmentation
|
Details
|
|
By Treatment Modality
|
Cell-Based Therapies, Non-Cell-Based Therapies
|
|
By Treatment Procedure
|
Palliative, Intrinsic Repair Stimulus
|
|
By Application
|
Knee, Hip, Ankle & Foot, Others
|
|
By End-User
|
Hospitals & Clinics, Ambulatory Surgical Centers
|
|
By Region
|
North America: U.S., Canada, Mexico
|
|
Europe: France, UK, Spain, Germany, Italy, Russia, Rest of Europe
|
|
Asia-Pacific: China, Japan, India, Australia, ASEAN, South Korea, Rest of Asia-Pacific
|
|
Middle East & Africa: Turkey, U.A.E., Saudi Arabia, South Africa, Rest of Middle East & Africa
|
|
South America: Brazil, Argentina, Rest of South America
|
Market Segmentation
- By Treatment Modality (Cell-Based Therapies and Non-Cell-Based Therapies): The non-cell-based therapies segment earned USD 3.45 billion in 2024, mainly due to widespread adoption of cost-effective synthetic implants and biomaterials for cartilage repair.
- By Treatment Procedure (Palliative and Intrinsic Repair Stimulus): The palliative segment held a share of 57.32% in 2024, fueled by the high prevalence of osteoarthritis and the rising need for symptom relief among patients unsuitable for surgical intervention.
- By Application (Knee, Hip, Ankle & Foot, and Others): The knee segment is projected to reach USD 4.71 billion by 2032, owing to the high incidence of knee injuries and degenerative joint conditions across all age groups.
- By End-User (Hospitals & Clinics and Ambulatory Surgical Centers): The ambulatory surgical centers segment is anticipated to witness the fastest CAGR of 6.55% over the forecast period, attributed to increasing preference for outpatient, minimally invasive cartilage repair procedures.
Global Cartilage Repair Market Regional Analysis
Based on region, the market has been classified into North America, Europe, Asia Pacific, Middle East & Africa, and South America.
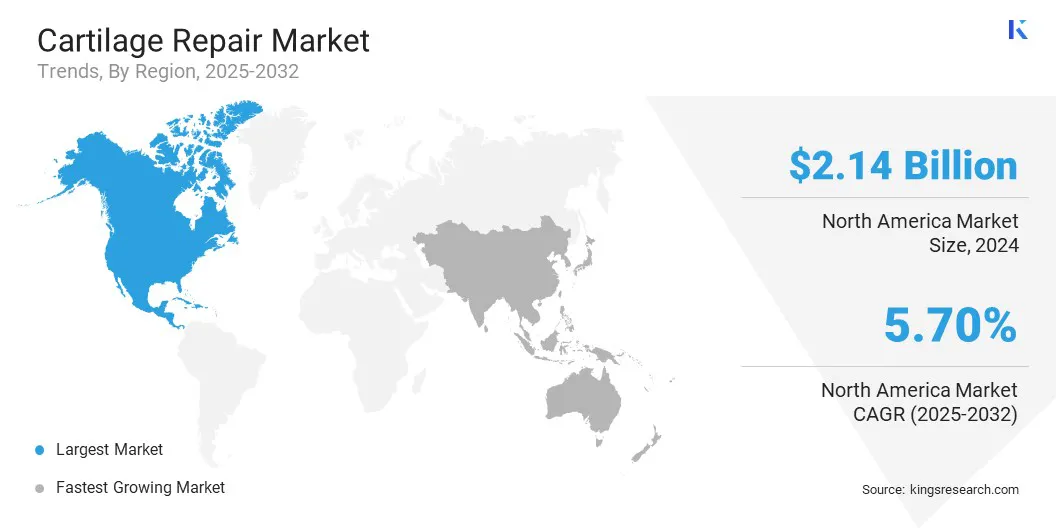
North America cartilage repair market share stood at 40.11% in 2024, valued at USD 2.14 billion. This dominance is primarily reinforced by the rising prevalence of osteoarthritis and sports-related joint injuries, which are increasing the demand for effective cartilage restoration therapies.
Technological advancements in regenerative medicine, including cell-based therapies and tissue-engineered scaffolds, are improving treatment outcomes and reducing recovery times for patients with damaged or degenerated joint cartilage.
The rising adoption of minimally invasive surgical techniques, such as arthroscopy and microfracture procedures, is boosting patient preference for cartilage repair procedures over traditional joint replacement. Additionally, growing R&D investments by pharmaceutical and biotech companies are fostering the introduction of novel therapies, stimulating regional market expansion.
- In August 2024, Vericel launched MACI Arthro, a biologic cartilage repair product, following FDA approval of a supplemental Biologics License Application. Designed for arthroscopic delivery of autologous cultured chondrocytes on a porcine collagen membrane, it enables repair of full-thickness knee cartilage defects. It provides a less invasive treatment option while maintaining the established clinical benefits of MACI.
The Asia-Pacific cartilage repair industry is set to grow at a CAGR of 6.49% over the forecast period. This growth is attributed to the surging geriatric population and the increasing prevalence of osteoarthritis and age-related joint disorders. Rising incidence of sports and lifestyle-related joint disorders in emerging economies such as China and India is boosting demand for effective cartilage repair therapies.
Increasing investment by governments and key players in regenerative medicine research for cartilage repair procedures in countries such as Japan, South Korea, and China is improving patient access to advanced regenerative treatments. Additionally, regulatory approvals of cartilage therapies are boosting the adoption of advanced repair procedures and fostering hospital investments, thereby supporting regional market growth.
- In May 2025, Japan Tissue Engineering Co., Ltd. received approval to expand the indication of its autologous cultured cartilage product, JACC, for treating osteoarthritis and knee cartilage damage.
Regulatory Frameworks
- In the U.S., the Food and Drug Administration (FDA) regulates cartilage repair products, including biologics, tissue-engineered therapies, and medical devices used for cartilage regeneration. It oversees clinical trial approvals, safety, efficacy, labeling, post-market surveillance, and adverse event reporting. The FDA ensures patient safety while promoting innovation in regenerative medicine.
- In the UK, the Medicines and Healthcare Products Regulatory Agency (MHRA) governs advanced therapy medicinal products (ATMPs) for cartilage repair, including cell and gene therapies. It oversees clinical trials, product licensing, safety, quality, and efficacy assessment, manufacturing standards, pharmacovigilance, and adverse event reporting. MHRA ensures that innovative regenerative treatments comply with strict safety and effectiveness standards.
- In China, the National Medical Products Administration (NMPA) regulates cartilage repair therapies, including autologous and allogeneic cell products, implants, and scaffolds. It supervise product registration, clinical trial approvals, safety standards, efficacy evaluation, manufacturing quality control, post-market monitoring, and adverse reaction reporting. NMPA aims to ensure safe and effective regenerative treatments for the growing orthopedic patient population.
- In India, the Central Drugs Standard Control Organization (CDSCO) regulates cartilage repair products, including cell-based therapies, tissue scaffolds, and implants. It supervise clinical trial approvals, product licensing, safety and efficacy evaluation, manufacturing compliance, quality control, and post-market surveillance to ensure regenerative therapies meet national standards and remain safe for patients.
Competitive Landscape
Key players in the cartilage repair industry are acquiring innovative technologies to expand treatment offerings and gain access to proprietary cartilage repair solutions. They are focusing on developing one-step, minimally invasive procedures for osteochondral lesions and early-stage osteoarthritis to enhance patient convenience and reduce recovery times for individuals requiring knee cartilage repair.
Additionally, firms are strengthening market presence by increasing product availability, expanding into new regions, and supporting healthcare professionals in adopting advanced cartilage repair procedures.
- In January 2024, Smith+Nephew acquired CartiHeal to gain Agili-C, a one-step cartilage repair technology for treating osteochondral lesions in the knee, including patients with mild to moderate osteoarthritis.
Key Companies in Cartilage Repair Market:
- Vericel Corporation
- Zimmer Biomet Holdings, Inc
- Smith+Nephew
- Arthrex, Inc
- Stryker Corporation
- Braun SE
- CONMED Corporation
- Anika Therapeutics, Inc
- MEDIPOST Co., Ltd
- Geistlich Pharma AG
- Orthocell Ltd
- Allosource
- Collagen Solutions Ltd
- Sparta Biomedical Inc
- TissueForm, Inc.
Recent Developments (M&A/Product Launch)
- In June 2025, OrthoCellix and Carisma Therapeutics entered into a definitive merger agreement to form a Nasdaq-listed company focused on regenerative cell therapies for orthopedic diseases. The merger aims to advance OrthoCellix’s Phase 3-ready NeoCart, an autologous cartilage implant technology for knee articular cartilage defects, with plans to initiate an FDA-endorsed Phase 3 clinical trial.
- In August 2024, Northwestern University developed a new bioactive material that mimics natural cartilage to promote cartilage repair. This offers potential in treating sports injuries, managing degenerative conditions, and reducing the need for joint replacements.


