Enquire Now
Cardiac Safety Services Market Size, Share, Growth & Industry Analysis, By Type (Integrated, Standalone), By Service Type (ECG / Holter Measurement, Blood Pressure Monitoring, Cardiovascular Imaging, Others), By End Use (Pharma & Biopharma Companies, Contract Research Organizations, Others), and Regional Analysis, 2025-2032
Pages: 170 | Base Year: 2024 | Release: September 2025 | Author: Versha V.
Key strategic points
Cardiac safety services are specialized preclinical and clinical testing services designed to assess the cardiovascular risks of pharmaceutical compounds, medical devices, or biologics.
They evaluate drug-induced effects on heart rate, blood pressure, QT interval, arrhythmia, and other cardiac parameters to ensure patient safety and regulatory compliance. The market encompasses a broad range of offerings, including ECG/Holter monitoring, blood pressure monitoring and cardiovascular imaging.
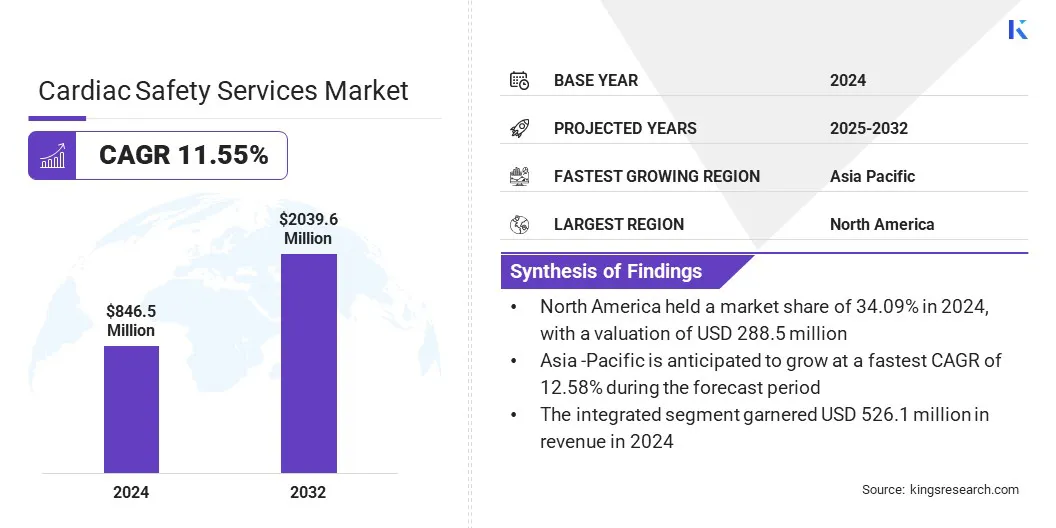
The global cardiac safety services market size was valued at USD 846.5 million in 2024 and is projected to grow from USD 941.9 million in 2025 to USD 2,039.6 million by 2032, exhibiting a CAGR of 11.55% during the forecast period.
Expanding drug development pipelines across multiple therapeutic areas are fueling the demand for cardiac safety assessments to meet regulatory and safety requirements. The rising prevalence of cardiovascular diseases globally is creating a significant demand for advanced cardiac safety services to ensure patient well-being during clinical trials.
Key Highlights:
Major companies operating in the cardiac safety services market are ICON plc, Clario, IQVIA Inc, Medpace, Inc, Charles River Laboratories, Eurofins Discovery Services LLC, Certara, Inc, Biotrial, Celerion, Richmond Pharmacology Limited, Ncardia Services B.V, Koninklijke Philips N.V, NEXEL Co., Ltd., Clario, and Laboratory Corporation of America Holdings.
Increasing investment by market players in AI-powered cardiac diagnostics is enabling earlier and more accurate detection of complex heart conditions. This advancement is improving patient outcomes and fueling the adoption of advanced cardiac safety services. In addition, AI-driven platforms are streamlining data interpretation, reducing human error, and accelerating decision-making in clinical trials.
Increasing Prevalence of Chronic Diseases
A key factor propelling the growth of the cardiac safety services market is the increasing prevalence of chronic diseases such as diabetes, hypertension, and obesity, which elevate the risk of cardiovascular complications. Pharmaceutical companies are prioritizing the development of safer therapies for these high-risk populations, prompting regulatory authorities to mandate comprehensive cardiac safety assessments during drug development.
Clinical trials are increasingly incorporating advanced monitoring technologies such as electrocardiogram (ECG) monitoring, Holter monitoring, and cardiac biomarker analysis to detect potential side effects early, reduce adverse events, and ensure patient safety. This growing focus on cardiovascular risk mitigation is boosting demand for specialized cardiac safety services.
High Cost of Cardiac Safety Evaluation Services
A key challenge impeding the growth of the cardiac safety services market is the high cost of cardiac safety evaluation services. These assessments require advanced monitoring equipment, sophisticated data analysis tools, and highly skilled professionals to ensure accurate and reliable results.
Extended trial durations, integration of multiple study endpoints, and large patient populations further increase operational expenses for cardiac safety evaluation services. Additionally, compliance with stringent regulatory standards adds to financial and administrative burdens, creating barriers for smaller companies and limiting broader adoption.
To address this challenge, market players are increasingly investing in advanced digital platforms, AI-driven analytics, and centralized monitoring solutions to streamline study processes and reduce operational expenses.
They are also forming strategic partnerships, collaborations, and acquisitions to share resources and expertise, enabling more efficient trial execution. Additionally, they are adopting remote and wearable cardiac monitoring technologies, which minimize the need for frequent patient visits and extensive on-site infrastructure.
Growing Adoption of Artificial Intelligence and Predictive Analytics in Cardiac Care
A key trend influencing the cardiac safety services market is the growing adoption of artificial intelligence and predictive analytics in cardiac care. AI-powered tools are enabling healthcare providers to predict cardiovascular risks with greater accuracy, facilitate early interventions, and deliver more personalized patient management.
These technologies are streamlining clinical decision-making, enhancing the efficiency of cardiac monitoring, and improving patient outcomes in both clinical trials and routine care.
|
Segmentation |
Details |
|
By Type |
Integrated, Standalone |
|
By Service Type |
ECG / Holter Measurement, Blood Pressure Monitoring, Cardiovascular Imaging, Thorough QT (TQT) Studies, Others |
|
By End Use |
Pharma & Biopharma Companies, Contract Research Organizations, Others |
|
By Region |
North America: U.S., Canada, Mexico |
|
Europe: France, UK, Spain, Germany, Italy, Russia, Rest of Europe |
|
|
Asia-Pacific: China, Japan, India, Australia, ASEAN, South Korea, Rest of Asia-Pacific |
|
|
Middle East & Africa: Turkey, U.A.E., Saudi Arabia, South Africa, Rest of Middle East & Africa |
|
|
South America: Brazil, Argentina, Rest of South America |
Based on region, the market has been classified into North America, Europe, Asia Pacific, Middle East & Africa, and South America.
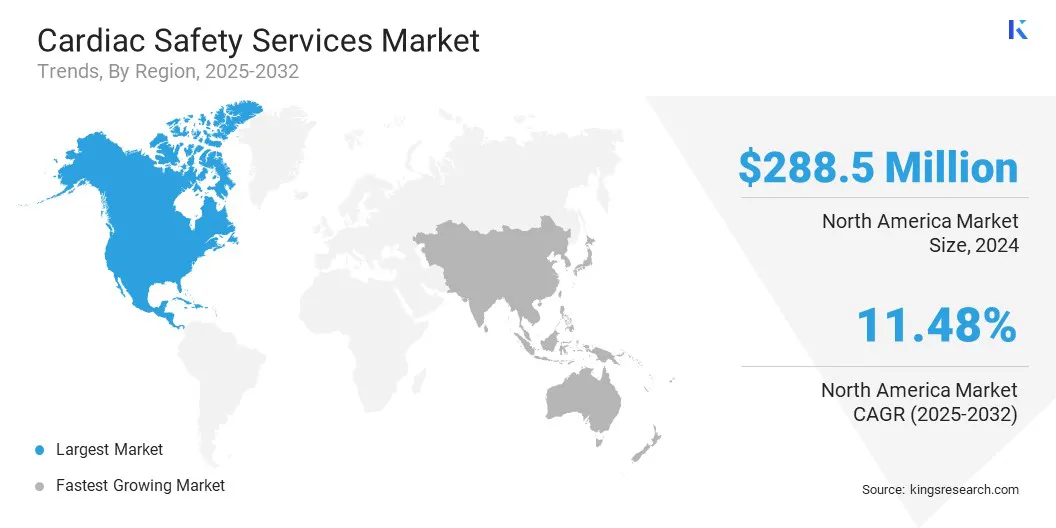
North America cardiac safety services market share stood at 34.09% in 2024, valued at USD 288.5 million. This dominance is reinforced by the rising prevalence of chronic diseases such as diabetes, hypertension, and obesity, which significantly increases the risk of cardiovascular complications.
Increasing regulatory scrutiny from agencies such as the U.S. Food and Drug Administration (FDA) is further compelling pharmaceutical companies to conduct comprehensive cardiac safety evaluations during drug development. Expanding drug development pipelines and a high volume of clinical trials across therapeutic areas are also fueling demand for specialized cardiac monitoring solutions.
The rapid adoption of advanced technologies, including electrocardiogram (ECG) analysis, wearable cardiac sensors, and in silico modeling, is enhancing the accuracy and efficiency of cardiac safety assessments. Additionally, growing investments in clinical trial services are strengthening service provider’s capabilities, enabling integrated, data-driven solutions for complex trials, thereby fueling regional market expansion.
The Asia-Pacific cardiac safety services industry is set to grow at a robust CAGR of 12.58% over the forecast period. This growth is attributed to the increasing cardiovascular disease prevalence, fueled by rapid urbanization and aging populations. Increasing government investments in healthcare infrastructure and clinical research initiatives to expand access to advanced medical technologies and strengthen trial capabilities are further boosting the need for cardiac safety services.
Additionally, the adoption of innovative cardiac care and monitoring platforms such as AI-powered risk prediction tools, wearable ECG devices, and advanced ablation systems is enhancing procedural accuracy and safety. This is leading to the widespread adoption of advanced cardiac safety services across the region, thereby fostering regional market growth.
Major players operating in the cardiac safety services industry are expanding their capabilities through partnerships with digital health technology providers to integrate wearable ECG devices into clinical trials. They are enhancing remote monitoring solutions to improve patient compliance, streamline data collection, and ensure accurate detection of cardiac events.
Additionally, market participants are advancing the use of AI-driven analytics, extended monitoring technologies, and digital endpoints to deliver high-quality cardiac safety data, improve trial efficiency, and reinforce their competitive market positioning.
Frequently Asked Questions