Market Definition
The market encompasses the diagnosis, treatment, and management of acute sinus infections, which are typically caused by viral, bacterial, or fungal pathogens. This market includes a range of pharmaceutical products such as antibiotics, decongestants, antihistamines, corticosteroids, and pain relievers, as well as medical devices like nasal irrigation systems and diagnostic tools.
The report provides insights into the fundamental drivers steering market growth, complemented by a thorough evaluation of market trends and the regulatory frameworks governing industry operations.
Acute Sinusitis Market Overview
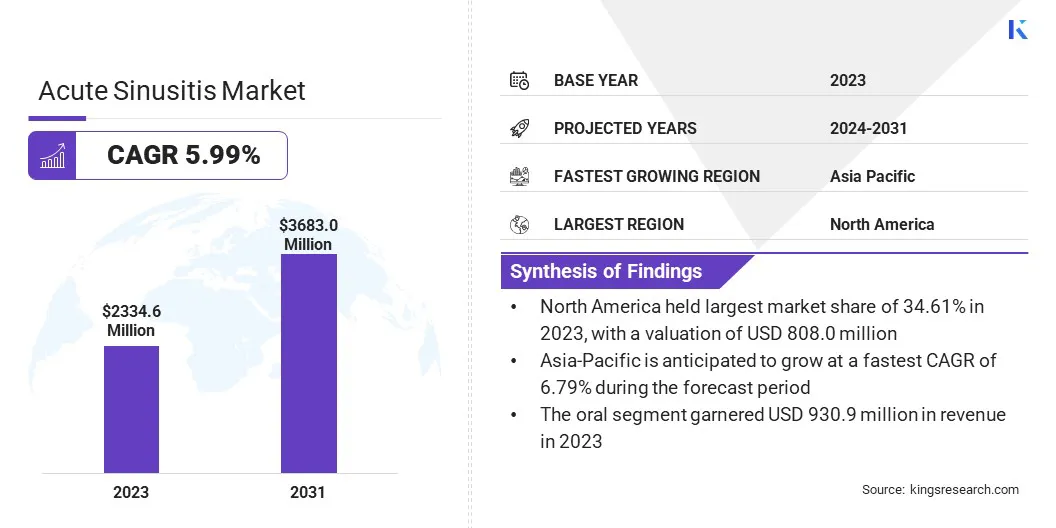
The global acute sinusitis market size was valued at USD 2334.6 million in 2023 and is projected to grow from USD 2451.1 million in 2024 to USD 3683.0 million by 2031, exhibiting a CAGR of 5.99% during the forecast period.
The market is driven by the rising prevalence of respiratory infections, increasing awareness of available treatment options, and advancements in diagnostic technologies.
Major companies operating in the acute sinusitis industry are Bayer AG, AstraZeneca, Dr. Reddy’s Laboratories Ltd., Novartis AG, GSK plc, Fresenius Kabi India Pvt. Ltd., Sanofi, weefselpharma.com., Kenvue Brands LLC., ANISH CHEMICALS, ADDII BIOTECH PVT LTD, Medo House, USAntibiotics, Medicef Pharma, and Hema Pharma.
The expanding adoption of both over-the-counter (OTC) and prescription medications, coupled with the growing integration of telemedicine and digital healthcare solutions, is further propelling the market.
North America leads the market, due to its robust healthcare infrastructure and strong pharmaceutical presence, while the market in Asia-Pacific is expected to grow rapidly due to rising healthcare investments and improved treatment access.
- In February 2024, SaNOtize Research & Development Corp. announced plans to evaluate its Nitric Oxide Nasal Spray (NONS) for the treatment of sinusitis. The study aims to assess the spray’s effectiveness in reducing inflammation and infection in patients with acute and chronic sinusitis.
Key Highlights
- The acute sinusitis industry size was valued at USD 2334.6 million in 2023.
- The market is projected to grow at a CAGR of 5.99% from 2024 to 2031.
- North America held a market share of 34.61% in 2023, with a valuation of USD 808.0 million.
- The antibiotic segment garnered USD 916.9 million in revenue in 2023.
- The oral segment is expected to reach USD 1481.3 million by 2031.
- The retail pharmacies & drug stores segment is anticipated to register the fastest CAGR of 6.57% during the forecast period.
- The market in Asia Pacific is anticipated to grow at a CAGR of 6.79% over the forecast period.
Market Driver
"Rising Prevalence of Respiratory Infections"
The acute sinusitis market is registering robust growth, propelled by the rising prevalence of respiratory infections such as the common cold and influenza. Environmental factors, including increasing air pollution and seasonal allergens, further contribute to the growing incidence of sinus-related conditions.
Additionally, factors such as urbanization, evolving lifestyle patterns, and compromised immune function due to stress or underlying health conditions have increased vulnerability to acute sinusitis.
The growing global prevalence of respiratory diseases, particularly in densely populated areas, has intensified the demand for advanced diagnostic technologies and effective treatment solutions, further driving the market.
- In March 2024, the U.S. Food and Drug Administration (FDA) granted approval for XHANCE (fluticasone propionate) nasal spray for the treatment of chronic rhinosinusitis (CRS) without nasal polyps in adult patients. This approval makes XHANCE the first and only medication indicated for this condition, addressing a significant unmet need for approximately 30 million affected individuals in the U.S.
Market Challenge
"Misdiagnosis and Underdiagnosis"
The growth of the acute sinusitis market is limited by misdiagnosis and underdiagnosis, as overlapping symptoms with other respiratory conditions often lead to incorrect or delayed treatment.
The lack of definitive diagnostic tests, combined with variations in symptom presentation, often leads to incorrect or delayed diagnoses, resulting in ineffective treatment and prolonged patient discomfort.
Inadequate awareness among patients and healthcare providers, especially in developing regions, exacerbates the issue. Many individuals self-medicate with OTC decongestants and pain relievers without seeking medical advice, which further contributes to underdiagnosis.
Advancements in diagnostic technologies, increased awareness, and improved clinical training are essential. Enhanced imaging, point-of-care testing, and AI-driven diagnostics can improve accuracy, while public health campaigns promote early recognition and medical consultation.
Telemedicine integration can further enhance accessibility, particularly in underserved areas. Standardizing treatment guidelines and refining physician training will ensure accurate diagnosis and effective management, ultimately improving patient outcomes and supporting market growth.
Market Trend
"Advancements in Diagnostic Technologies"
Advancements in diagnostic technologies are significantly improving the accuracy and efficiency of acute sinusitis detection and management. Innovations such as AI-powered imaging, point-of-care testing, and biomarker-based diagnostics enable faster and more precise differentiation between viral and bacterial sinus infections, reducing the risk of misdiagnosis.
AI-driven radiology tools enhance the interpretation of CT scans, while rapid diagnostic tests allow for early intervention and targeted treatment. Additionally, the integration of digital health solutions, including telemedicine and mobile health applications, is enhancing accessibility to diagnostic services, particularly in remote areas.
- In May 2024, a reseach team led by the Indiana University School of Medicine and supported by a USD 3.5 million NIAID grant, developed an AI-driven prediction model to assess surgical outcomes for chronic rhinosinusitis patients. This initiative aims to enhance personalized treatment and reduce unnecessary sinus surgeries.
Acute Sinusitis Market Report Snapshot
|
Segmentation
|
Details
|
|
By Treatment Type
|
Antibiotic, Analgesic, Anti-inflammatory, Expectorants
|
|
By Route of Administration
|
Nasal, Oral, Injectable, Topical
|
|
By End User
|
Hospitals, Clinics, Retail Pharmacies & Drug Stores, Online Pharmacies
|
|
By Region
|
North America: U.S., Canada, Mexico
|
|
Europe: France, UK, Spain, Germany, Italy, Russia, Rest of Europe
|
|
Asia-Pacific: China, Japan, India, Australia, ASEAN, South Korea, Rest of Asia-Pacific
|
|
Middle East & Africa: Turkey, UAE, Saudi Arabia, South Africa, Rest of Middle East & Africa
|
|
South America: Brazil, Argentina, Rest of South America
|
Market Segmentation
- By Treatment Type (Antibiotic, Analgesic, Anti-inflammatory, Expectorants): The antibiotic segment earned USD 916.9 million in 2023, due to its widespread use as the primary treatment for bacterial acute sinusitis and high prescription rates by healthcare providers.
- By Route of Administration (Nasal, Oral, Injectable, Topical): The oral segment held 39.88% share of the market in 2023, due to its convenience, high patient compliance, and widespread availability of oral antibiotics, analgesics, and anti-inflammatory medications.
- By End User (Hospitals, Clinics, Retail Pharmacies & Drug Stores, Online Pharmacies): The hospitals segment is projected to reach USD 1469.9 million by 2031, owing to the rising number of hospital visits for severe and recurrent cases, availability of advanced diagnostic and treatment facilities, and increasing preference for specialist consultations.
Acute Sinusitis Market Regional Analysis
Based on region, the market has been classified into North America, Europe, Asia Pacific, Middle East & Africa, and South America.
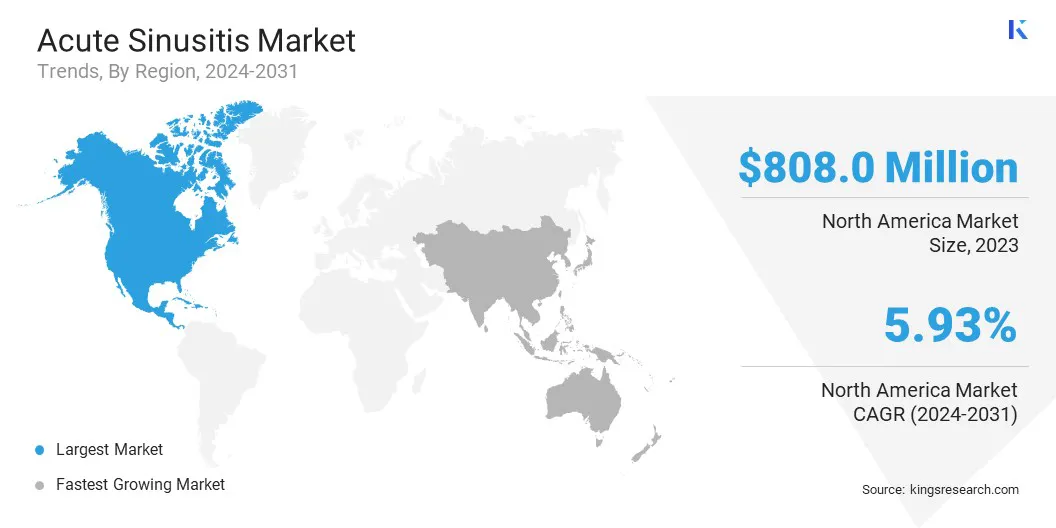
North America acute sinusitis market share stood at around 34.61% in 2023, with a valuation of USD 808.0 million. The dominance is primarily attributed to the high prevalence of respiratory infections, well-established healthcare infrastructure, and widespread availability of advanced treatment options.
The growth is further supported by increasing awareness about sinusitis management, coupled with favorable reimbursement policies and the presence of key pharmaceutical players. Furthermore, the increasing use of minimally invasive sinus procedures and continuous advancements in novel therapeutics are driving the market in North America.
- In December 2024, Endo, Inc. announced the launch of doxycycline for injection, USP 100 mg/vial, an AP-rated generic version of DOXY 100. This addition strengthens Endo's sterile injectables portfolio, offering healthcare providers a cost-effective alternative for treating various infections.
The acute sinusitis industry in Asia Pacific is expected to register the fastest CAGR of 6.79% over the forecast period. This growth is fueled by the rising prevalence of respiratory infections, increasing healthcare expenditure, and improving access to advanced medical treatments.
Rapid urbanization, worsening air pollution levels, and growing awareness about sinusitis management further support the market expansion. Moreover, the surging demand for OTC and prescription medications, along with the expansion of pharmaceutical companies in emerging economies like China and India, is contributing to the region’s accelerated market growth.
Regulatory Frameworks
- In Europe, the European Medicines Agency (EMA) regulates medicinal products under Regulation (EC) No 726/2004, establishing standards for the authorization, supervision, and pharmacovigilance of medicines to ensure safety, efficacy, and quality across EU member states.
- In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates medicinal products and medical devices, ensuring compliance with the Pharmaceuticals and Medical Devices Act (PMD Act) through rigorous evaluation, approval, and post-market surveillance to maintain safety, efficacy, and quality standards.
- In the U.S., the Food and Drug Administration (FDA) regulates acute bacterial sinusitis treatments under the Guidance for Industry: Acute Bacterial Sinusitis – Developing Drugs for Treatment, outlining standards for clinical trial design, efficacy evaluation, and safety assessment to ensure the approval of effective and high-quality therapies.
- In Brazil, the National Health Surveillance Agency (ANVISA) regulates medicinal products and medical devices, establishing standards for approval, quality control, and post-market surveillance to ensure the safety, efficacy, and compliance of healthcare products within the country.
Competitive Landscape
The acute sinusitis industry is characterized by intense competition among key players, driven by ongoing product innovations, strategic mergers and acquisitions, and expanding research initiatives.
Leading pharmaceutical and biotechnology companies are focusing on developing advanced treatment options, including novel antibiotics, corticosteroids, and minimally invasive procedures, to strengthen their market position. Furthermore, partnerships with healthcare providers and investments in digital health solutions are enhancing patient access to effective treatments.
- In May 2024, Dr. Reddy's Laboratories Ltd. launched Doxycycline Capsules, 40 mg in the U.S., a therapeutic generic equivalent of ORACEA (doxycycline, USP) capsules, 40 mg, following approval from the U.S. FDA.
List of Key Companies in Acute Sinusitis Market:
- Bayer AG
- AstraZeneca
- Reddy’s Laboratories Ltd.
- Novartis AG
- GSK plc
- Fresenius Kabi India Pvt. Ltd.
- Sanofi
- weefselpharma.com.
- Kenvue Brands LLC.
- ANISH CHEMICALS
- ADDII BIOTECH PVT LTD
- Medo House
- USAntibiotics
- Medicef Pharma
- Hema Pharma
Recent Developments (M&A/Partnerships/Agreements/Product Launches)
- In March 2025, Paratek Pharmaceuticals, Inc announced a definitive merger agreement to acquire Optinose, Inc., including its approved product XHANCE, in a deal valued at up to $330 million. This acquisition expands Paratek’s portfolio beyond its flagship antibiotic NUZYRA, reinforcing its position as a multi-product company specializing in innovative therapies for primary care and specialty providers.
- In October 2024, GSK plc announced positive Phase III ANCHOR trial results for depemokimab in chronic rhinosinusitis with nasal polyps (CRSwNP), showing significant reductions in polyp size and nasal obstruction.
- In September 2024, Regeneron Pharmaceuticals, Inc. and Sanofi announced the FDA-approved Dupixent (dupilumab) as an add-on maintenance treatment for adolescents aged 12 to 17 years with inadequately controlled chronic rhinosinusitis with nasal polyps (CRSwNP).


